The Susceptibility of Chickens to Zika Virus: A Comprehensive Study on Age-Dependent Infection Dynamics and Host Responses
- PMID: 38675911
- PMCID: PMC11054531
- DOI: 10.3390/v16040569
The Susceptibility of Chickens to Zika Virus: A Comprehensive Study on Age-Dependent Infection Dynamics and Host Responses
Abstract
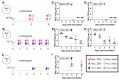
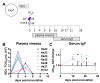
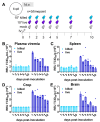
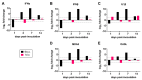
Zika virus (ZIKV) remains a public health concern, with epidemics in endemic regions and sporadic outbreaks in new areas posing significant threats. Several mosquito-borne flaviviruses that can cause human illness, including West Nile, Usutu, and St. Louis encephalitis, have associations with birds. However, the susceptibility of chickens to ZIKV and their role in viral epidemiology is not currently known. We investigated the susceptibility of chickens to experimental ZIKV infection using chickens ranging from 1-day-old chicks to 6-week-old birds. ZIKV caused no clinical signs in chickens of all age groups tested. Viral RNA was detected in the blood and tissues during the first 5 days post-inoculation in 1-day and 4-day-old chicks inoculated with a high viral dose, but ZIKV was undetectable in 6-week-old birds at all timepoints. Minimal antibody responses were observed in 6-week-old birds, and while present in younger chicks, they waned by 28 days post-infection. Innate immune responses varied significantly between age groups. Robust type I interferon and inflammasome responses were measured in older chickens, while limited innate immune activation was observed in younger chicks. Signal transducer and activator of transcription 2 (STAT2) is a major driver of host restriction to ZIKV, and chicken STAT2 is distinct from human STAT2, potentially contributing to the observed resistance to ZIKV infection. The rapid clearance of the virus in older chickens coincided with an effective innate immune response, highlighting age-dependent susceptibility. Our study indicates that chickens are not susceptible to productive ZIKV infection and are unlikely to play a role in the ZIKV epidemiology.
Keywords: STAT2; Zika virus; antibody; chicken; host restriction; innate immune response.
Conflict of interest statement
Levina Lim is currently employed by DermBiont, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Activation of ATF3 via the integrated stress response pathway regulates innate immune response to restrict Zika virus.J Virol. 2024 Oct 22;98(10):e0105524. doi: 10.1128/jvi.01055-24. Epub 2024 Aug 30. J Virol. 2024. PMID: 39212382 Free PMC article.
-
Genetic Diversity of Collaborative Cross Mice Controls Viral Replication, Clinical Severity, and Brain Pathology Induced by Zika Virus Infection, Independently of Oas1b.J Virol. 2020 Jan 17;94(3):e01034-19. doi: 10.1128/JVI.01034-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694939 Free PMC article.
-
Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface.J Virol. 2017 Jan 31;91(4):e01905-16. doi: 10.1128/JVI.01905-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974560 Free PMC article.
-
Taking the defensive: Immune control of Zika virus infection.Virus Res. 2018 Aug 2;254:21-26. doi: 10.1016/j.virusres.2017.08.018. Epub 2017 Sep 1. Virus Res. 2018. PMID: 28867493 Free PMC article. Review.
-
Immune Response to Dengue and Zika.Annu Rev Immunol. 2018 Apr 26;36:279-308. doi: 10.1146/annurev-immunol-042617-053142. Epub 2018 Jan 18. Annu Rev Immunol. 2018. PMID: 29345964 Free PMC article. Review.
Cited by
-
Advances in Alphavirus and Flavivirus Research.Viruses. 2024 May 30;16(6):882. doi: 10.3390/v16060882. Viruses. 2024. PMID: 38932175 Free PMC article.
References
-
- Postler T.S., Beer M., Blitvich B.J., Bukh J., de Lamballerie X., Drexler J.F., Imrie A., Kapoor A., Karganova G.G., Lemey P., et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023;168:224. doi: 10.1007/s00705-023-05835-1. - DOI - PubMed
-
- Brasil P., Calvet G.A., Siqueira A.M., Wakimoto M., de Sequeira P.C., Nobre A., Quintana Mde S., Mendonca M.C., Lupi O., de Souza R.V., et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl. Trop. Dis. 2016;10:e0004636. doi: 10.1371/journal.pntd.0004636. - DOI - PMC - PubMed
-
- Miranda-Filho Dde B., Martelli C.M., Ximenes R.A., Araújo T.V., Rocha M.A., Ramos R.C., Dhalia R., França R.F., Marques Júnior E.T., Rodrigues L.C. Initial Description of the Presumed Congenital Zika Syndrome. Am. J. Public Health. 2016;106:598–600. doi: 10.2105/AJPH.2016.303115. - DOI - PMC - PubMed
-
- França G.V., Schuler-Faccini L., Oliveira W.K., Henriques C.M., Carmo E.H., Pedi V.D., Nunes M.L., Castro M.C., Serruya S., Silveira M.F., et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

