Combining Cellular Immunization and Phage Display Screening Results in Novel, FcγRI-Specific Antibodies
- PMID: 38675937
- PMCID: PMC11053525
- DOI: 10.3390/v16040596
Combining Cellular Immunization and Phage Display Screening Results in Novel, FcγRI-Specific Antibodies
Abstract
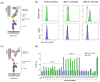
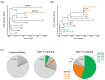
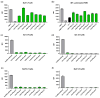
Antibodies that specifically bind to individual human fragment crystallizable γ receptors (FcγRs) are of interest as research tools in studying immune cell functions, as well as components in bispecific antibodies for immune cell engagement in cancer therapy. Monoclonal antibodies for human low-affinity FcγRs have been successfully generated by hybridoma technology and are widely used in pre-clinical research. However, the generation of monoclonal antibodies by hybridoma technology that specifically bind to the high-affinity receptor FcγRI is challenging. Monomeric mouse IgG2a, IgG2b, and IgG3 bind human FcγRI with high affinity via the Fc part, leading to an Fc-mediated rather than a fragment for antigen binding (Fab)-mediated selection of monoclonal antibodies. Blocking the Fc-binding site of FcγRI with an excess of human IgG or Fc during screening decreases the risk of Fc-mediated interactions but can also block the potential epitopes of new antibody candidates. Therefore, we replaced hybridoma technology with phage display of a single-chain fragment variable (scFv) antibody library that was generated from mice immunized with FcγRI-positive cells and screened it with a cellular panning approach assisted by next-generation sequencing (NGS). Seven new FcγRI-specific antibody sequences were selected with this methodology, which were produced as Fc-silent antibodies showing FcγRI-restricted specificity.
Keywords: ELISA; Fc receptor; FcγRI; NGS; cellular panning; phage display.
Conflict of interest statement
S.K., T.H., A.M.B., J.H.W.L. and M.P. are inventors of related patent applications. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- van der Poel C.E., Karssemeijer R.A., Boross P., van der Linden J.A., Blokland M., van de Winkel J.G., Leusen J.H. Cytokine-Induced Immune Complex Binding to the High-Affinity Igg Receptor, Fcgammari, in the Presence of Monomeric Igg. Blood. 2010;116:5327–5333. doi: 10.1182/blood-2010-04-280214. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

