Risk of Cardiovascular Events in People with HIV (PWH) Treated with Integrase Strand-Transfer Inhibitors: The Debate Is Not Over; Results of the SCOLTA Study
- PMID: 38675955
- PMCID: PMC11054557
- DOI: 10.3390/v16040613
Risk of Cardiovascular Events in People with HIV (PWH) Treated with Integrase Strand-Transfer Inhibitors: The Debate Is Not Over; Results of the SCOLTA Study
Abstract
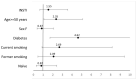
Cardiovascular disease (CVD) is common in people with HIV (PWH), and has great impact in terms of morbidity and mortality. Several intertwined mechanisms are believed to play a role in determining the increased risk of CVD, including the effect of certain antiretrovirals; among these, the role of integrase strand-transfer inhibitors (INSTIs) is yet to be fully elucidated. We conducted a multicenter, observational study comprising 4984 PWH evaluating the antiretroviral therapy (ART)-related nature of CVD in real life settings, both in naïve vs. treatment-experienced people. A comparison was conducted between INSTIs vs. either protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs) considering demographic, baseline clinical characteristics, incidence of CVD in both 2-year and complete follow-up periods. Among 2357 PWH exposed to INSTIs, 24 people experienced CVD; the corresponding figure was 12 cases out of 2599 PWH exposed to other ART classes. At univariate and multivariate analysis, a tendency towards an increased risk of CVD was observed in the 2-year follow-up period in PWH exposed to INSTIs in the absence, however, of statistical significance. These findings leave open the hypothesis that INSTIs may play a role, albeit minimal, in determining an increased risk of CVD in PWH.
Keywords: HIV; cardiovascular disease; integrase strand transfer inhibitors; observational study.
Conflict of interest statement
P.M. received consultancy and/or speakers’ fees from Gilead, ViiV, Merck Sharp & Dohme, and Janssen; G.V.D.S. received speaker’s honoraria from Gilead and ViiV Healthcare; G.M. received consultancy and/or speakers’ fees from Astra Zeneca, Gilead Sciences, Glaxo SmithKline, Janssen, Merck Sharp & Dohme, Thera technologies and ViiV Healthcare; M.A.C. received consultancy and/or speakers’ fees from ViiV Healthcare, Gilead, Janssen, and Merck Sharp & Dohme; L.T. attended advisory boards or served as consultant or received grants for conference participations from Gilead Sciences, ViiV Healthcare, and Janssen; A.D.B. received funding for scientific advisory boards, travel, and speaker honoraria from ViiV Healthcare, Gilead, Janssen, Abbvie, Merck Sharp & Dohme; P.B. attended advisory boards for Viiv Healthcare, Gilead, Merck Sharp & Dohme, Astrazeneca, Jannsen; N.S. received consultancy and/or speakers’ fees from Gilead Science and ViiV Healthcare; F.L. participated on advisory boards sponsored by ViiV Healthcare, Janssen and received educational and grant support from Gilead; B.M.C. reported honoraria for presentations and scientific advice from Gilead Sciences, ViiV Healthcare, Janssen-Cilag, Mylan, GSK vaccines and Merck Sharp & Dohme and research grants for his institution from Gilead Sciences, ViiV Healthcare GSK vaccines and Merck Sharp & Dohme; R.G. participated on advisory boards sponsored by ViiV Healthcare, Merck Sharp & Dohme, Gilead, and received speaker’s honoraria and/or support for travel meetings from Gilead, ViiV Healthcare, Merck Sharp & Dohme, AbbVie, and grant support from ViiV Healthcare and Gilead; A.C. served as consultant or advisory board and/or received speaker’s fees and/or received research grants for their institutions, outside the present work from Gilead Science, ViiV Healthcare, Janssen and Merck Sharp & Dohme; R.B. received speaker’s honoraria from Gilead Science, ViiV Healthcare, Janssen-Cilag and Merck Sharp & Dohme; A.B. received speaker’s honoraria and fees for attending advisory boards from Astra-Zeneca, BioMerieux, Janssen-Cilag, Nordic Pharma, Pfizer, Qiagen, SOBI, Takeda, ViiV and received research grants from Gilead; L.C. received speaker honoraria from Gilead, ViiV Healthcare, Merck and Janssen, and attended advisory boards of Gilead, ViiV Healthcare, Merck and Janssen; O.B. received speaker’s fees, consultancies and travel grants from Gilead, ViiV Healthcare and Janssen. All remaining authors have no potential conflicts of interest.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 2022. In Danger: UNAIDS Global AIDS Update 2022; 2022, 2022, 1–22. [(accessed on 11 April 2024)]. Available online: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids....
-
- World Health Organization . Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted in-Fections for the Period 2022–2030. World Health Organization; Geneva, Switzerland: 2022.