Co3O4-Based Materials as Potential Catalysts for Methane Detection in Catalytic Gas Sensors
- PMID: 38676216
- PMCID: PMC11054299
- DOI: 10.3390/s24082599
Co3O4-Based Materials as Potential Catalysts for Methane Detection in Catalytic Gas Sensors
Abstract
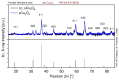
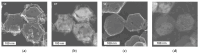
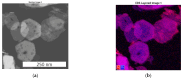
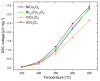
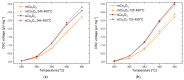
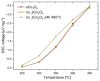
The present work deals with the development of Co3O4-based catalysts for potential application in catalytic gas sensors for methane (CH4) detection. Among the transition-metal oxide catalysts, Co3O4 exhibits the highest activity in catalytic combustion. Doping Co3O4 with another metal can further improve its catalytic performance. Despite their promising properties, Co3O4 materials have rarely been tested for use in catalytic gas sensors. In our study, the influence of catalyst morphology and Ni doping on the catalytic activity and thermal stability of Co3O4-based catalysts was analyzed by differential calorimetry by measuring the thermal response to 1% CH4. The morphology of two Co3O4 catalysts and two NixCo3-xO4 with a Ni:Co molar ratio of 1:2 and 1:5 was studied using scanning transmission electron microscopy and energy dispersive X-ray analysis. The catalysts were synthesized by (co)precipitation with KOH solution. The investigations showed that Ni doping can improve the catalytic activity of Co3O4 catalysts. The thermal response of Ni-doped catalysts was increased by more than 20% at 400 °C and 450 °C compared to one of the studied Co3O4 oxides. However, the thermal response of the other Co3O4 was even higher than that of NixCo3-xO4 catalysts (8% at 400 °C). Furthermore, the modification of Co3O4 with Ni simultaneously brings stability problems at higher operating temperatures (≥400 °C) due to the observed inhomogeneous Ni distribution in the structure of NixCo3-xO4. In particular, the NixCo3-xO4 with high Ni content (Ni:Co ratio 1:2) showed apparent NiO separation and thus a strong decrease in thermal response of 8% after 24 h of heat treatment at 400 °C. The reaction of the Co3O4 catalysts remained quite stable. Therefore, controlling the structure and morphology of Co3O4 achieved more promising results, demonstrating its applicability as a catalyst for gas sensing.
Keywords: catalyst; catalytic sensors; cobalt oxide; morphology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Bársony I., Ádám M., Fürjes P., Lucklum R., Hirschfelder M., Kulinyi S., Dücső C. Efficient catalytic combustion in integrated micropellistors. Meas. Sci. Technol. 2009;20:124009. doi: 10.1088/0957-0233/20/12/124009. - DOI
-
- Szulczyński B., Gębicki J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments. 2017;4:21. doi: 10.3390/environments4010021. - DOI
-
- Roslyakov I., Kolesnik I., Evdokimov P., Skryabina O., Garshev A., Mironov S., Stolyarov V., Baranchikov A., Napolskii K. Microhotplate catalytic sensors based on porous anodic alumina: Operando study of methane response hysteresis. Sens. Actuators B. 2021;330:129307. doi: 10.1016/j.snb.2020.129307. - DOI
-
- Samotaev N., Pisliakov A., Gorshkova A., Dzhumaev P., Barsony I., Ducso C., Biro F. Al2O3 nanostructured gas sensitive material for silicon based low power thermocatalytic sensor. Mater. Today Proc. 2020;30:443–447. doi: 10.1016/j.matpr.2019.12.393. - DOI
-
- Lorenzo-Bayona J.L., León D., Amez I., Castells B., Medic L. Experimental Comparison of Functionality between the Main Types of Methane Measurement Sensors in Mines. Energies. 2023;16:2207. doi: 10.3390/en16052207. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

