Proof-of-concept study of anti-Fel d 1 IgY antibodies in cat food using the MASK-air® app
- PMID: 38676659
- PMCID: PMC11055507
- DOI: 10.1002/clt2.12353
Proof-of-concept study of anti-Fel d 1 IgY antibodies in cat food using the MASK-air® app
Abstract
Background: An innovation to better manage cat-allergic patients utilises anti-Fel d 1 IgY antibodies to neutralise Fel d 1 after its production by the cat. However, there is no published study showing its clinical efficacy in humans in a home setting. A longitudinal, open-label, proof-of-concept study was carried out to approach clinical efficacy of the cat food in cat-allergic patients.
Methods: After a baseline evaluation, the cats ate only the cat food for the following 4 months. Daily evaluation of efficacy was performed for 2 weeks at baseline and after 1, 2 and 3 months of intervention for periods of 2 weeks. The MASK-air app was used daily to assess symptoms, work productivity and medications.
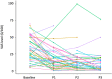
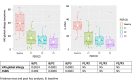
Results: Of the 49 patients screened, 42 were followed up and 33 (78.5%) reported MASK-air data at all 3 evaluation periods. The primary end point (visual analogue scale [VAS] for global allergy symptoms) was significantly improved (p < 0.0001). All symptoms (VAS nose, eye, and asthma), VAS work and the combined symptom-medication score significantly improved after 1 month. The percentage of uncontrolled days (VAS>20/100) decreased from 64% at baseline to 35% at 1 month (p < 0.0001) and 14% at 3 months. A sensitivity analysis in patients with uncontrolled disease at baseline found similar results.
Discussion: A cat diet containing anti-Fel d 1 antibodies was able to (i) show decreased allergic symptoms and related outcomes, (ii) inform the design and feasibility of future studies with a control arm and (iii) estimate the sample size of the study.
Study registration number: clinicaltrials.gov: NCT05656482.
Keywords: anti‐Fel d 1 antibodies; asthma; cat allergy; cat food; combined symptom‐medication score; conjunctivitis; rhinitis.
© 2024 The Authors. Clinical and Translational Allergy published by John Wiley and Sons Ltd on behalf of European Academy of Allergy and Clinical Immunology.
Conflict of interest statement
JB reports personal fees from Cipla, Menarini, Mylan, Novartis, Purina, Sanofi‐Aventis, Teva, Noucor, other from KYomed‐Innov, other from Mask‐air‐SAS, outside the submitted work. TZ reports grants and personal fees from Novartis, grants and personal fees from Henkel, personal fees from Bayer, personal fees from FAES, personal fees from Astra Zeneca, personal fees from AbbVie, personal fees from ALK, personal fees from Almirall, personal fees from Astellas, personal fees from Bayer, personal fees from Bencard, personal fees from Berlin Chemie, personal fees from FAES, personal fees from Hal, personal fees from Leti, personal fees from Mesa, personal fees from Menarini, personal fees from Merck, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Sanofi, personal fees from Stallergenes, personal fees from Takeda, personal fees from Teva, personal fees from UCB, personal fees from Henkel, personal fees from Kryolan, personal fees from L'Oreal, outside the submitted work; and Organisational affiliations: Commitee member: WHO‐Initiative "Allergic Rhinitis and Its Impact on Asthma” (ARIA); Member of the Board: German Society for Allergy and Clinical Immunology (DGAKI); Head: European Centre for Allergy Research Foundation (ECARF); President: Global Allergy and Asthma European Network (GA2LEN); Member: Committee on Allergy Diagnosis and Molecular Allergology, World Allergy Organisation (WAO). FdB reports other from NOVARTIS, other from ALK, other from STALLERGENES, other from REGENERON, other from DBV, other from SANOFI, other from BOEHRINGER, and other from ASTRAZENECA, outside the submitted work. The other authors have nothing to disclose, outside the submitted work.
Figures


References
-
- Shamji MH, Singh I, Layhadi JA, et al. Passive prophylactic administration with a single dose of anti‐fel d 1 monoclonal antibodies REGN1908‐1909 in cat allergen‐induced allergic rhinitis: a randomized, double‐blind, placebo‐controlled clinical trial. Am J Respir Crit Care Med. 2021;204(1):23‐33. 10.1164/rccm.202011-4107OC - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

