The e-liquid flavoring cinnamaldehyde induces cellular stress responses in human proximal tubule (HK-2) kidney cells
- PMID: 38677246
- PMCID: PMC11293278
- DOI: 10.1016/j.biopha.2024.116666
The e-liquid flavoring cinnamaldehyde induces cellular stress responses in human proximal tubule (HK-2) kidney cells
Abstract
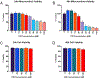
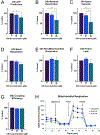
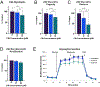
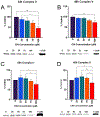
Flavored e-liquid use has become popular among e-cigarette users recently, but the effects of such products outside the lung are not well characterized. In this work, acute exposure to the popular flavoring cinnamaldehyde (CIN) was performed on human proximal tubule (HK-2) kidney cells. Cells were exposed to 0-100 µM CIN for 24-48 h and cellular stress responses were assessed. Mitochondrial viability via MTT assay was significantly decreased at 20 µM for 24 and 48 h exposure. Seahorse XFp analysis showed significantly decreased mitochondrial energy output at 20 µM by 24 h exposure, in addition to significantly reduced ATP Synthase expression. Seahorse analysis also revealed significantly decreased glycolytic function at 20 µM by 24 h exposure, suggesting inability of glycolytic processes to compensate for reduced mitochondrial energy output. Cleaved caspase-3 expression, a mediator of apoptosis, was significantly increased at the 24 h mark. C/EBP homologous protein (CHOP) expression, a mediator of ER-induced apoptosis, was induced by 48 h and subsequently lost at the highest concentration of 100 µM. This decrease was accompanied by a simultaneous decrease in its downstream target cleaved caspase-3 at the 48 h mark. The autophagy marker microtubule-associated protein 1 A/1B light chain 3 (LC3B-I and LC3B-II) expression was significantly increased at 100 µM by 24 h. Autophagy-related 7 (ATG7) protein and mitophagy-related proteins PTEN-induced putative kinase 1 (PINK1) and PARKIN expression were significantly reduced at 24 and 48 h exposure. These results indicate acute exposure to CIN in the kidney HK-2 model induces mitochondrial dysfunction and cellular stress responses.
Keywords: Cellular stress; Cinnamaldehyde; E-cigarette; Flavor; Kidney.
Copyright © 2024 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures




Similar articles
-
The E-liquid flavoring vanillin alters energy and autophagic pathways in human proximal tubule (HK-2) epithelial cells.Chem Biol Interact. 2024 May 1;394:111003. doi: 10.1016/j.cbi.2024.111003. Epub 2024 Apr 10. Chem Biol Interact. 2024. PMID: 38608998
-
The Flavoring Agent Ethyl Vanillin Induces Cellular Stress Responses in HK-2 Cells.Toxics. 2024 Jun 29;12(7):472. doi: 10.3390/toxics12070472. Toxics. 2024. PMID: 39058124 Free PMC article.
-
Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys.Autophagy. 2019 Dec;15(12):2142-2162. doi: 10.1080/15548627.2019.1615822. Epub 2019 May 22. Autophagy. 2019. PMID: 31066324 Free PMC article.
-
Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function.Am J Physiol Lung Cell Mol Physiol. 2019 Mar 1;316(3):L470-L486. doi: 10.1152/ajplung.00304.2018. Epub 2019 Jan 3. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30604630 Free PMC article.
-
Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation.Autophagy. 2019 Sep;15(9):1606-1619. doi: 10.1080/15548627.2019.1591672. Epub 2019 Apr 7. Autophagy. 2019. PMID: 30859901 Free PMC article.
Cited by
-
Flavoured Vaping Products in Tobacco Harm Reduction: A Regulatory Perspective.Cureus. 2025 Aug 1;17(8):e89196. doi: 10.7759/cureus.89196. eCollection 2025 Aug. Cureus. 2025. PMID: 40757083 Free PMC article. Review.
References
-
- Administration, Fa.D., FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children including fruit and mint.pdf. 2020.
-
- Miech RA, Johnston LD, Patrick ME, O’Malley PM, Bachman JG, Schulenberg JE, Monitoring the Future national survey results on drug use, 1975–2022: Secondary school students. Monitoring the Future Monograph Series. Ann Arbor, MI: Institute for Social Research, University of Michigan. Available at https://monitoringthefuture.org/results/publications/monographs/2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

