CD80 on skin stem cells promotes local expansion of regulatory T cells upon injury to orchestrate repair within an inflammatory environment
- PMID: 38677291
- PMCID: PMC11265648
- DOI: 10.1016/j.immuni.2024.04.003
CD80 on skin stem cells promotes local expansion of regulatory T cells upon injury to orchestrate repair within an inflammatory environment
Abstract
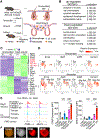
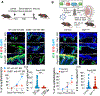
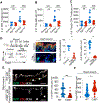
Following tissue damage, epithelial stem cells (SCs) are mobilized to enter the wound, where they confront harsh inflammatory environments that can impede their ability to repair the injury. Here, we investigated the mechanisms that protect skin SCs within this inflammatory environment. Characterization of gene expression profiles of hair follicle SCs (HFSCs) that migrated into the wound site revealed activation of an immune-modulatory program, including expression of CD80, major histocompatibility complex class II (MHCII), and CXC motif chemokine ligand 5 (CXCL5). Deletion of CD80 in HFSCs impaired re-epithelialization, reduced accumulation of peripherally generated Treg (pTreg) cells, and increased infiltration of neutrophils in wounded skin. Importantly, similar wound healing defects were also observed in mice lacking pTreg cells. Our findings suggest that upon skin injury, HFSCs establish a temporary protective network by promoting local expansion of Treg cells, thereby enabling re-epithelialization while still kindling inflammation outside this niche until the barrier is restored.
Keywords: CD80; Treg cell; hair follicle stem cell; neutrophils; wound repair.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.F. is on the editorial board of some Elsevier Journals (Cell, Cell Stem Cell, and Developmental Cell). A.Y.R. is a member of SAB and has equity in Coherus, RAPT Therapeutics, Sonoma Biotherapeutics, Santa Ana Bio, and Vedanta Biosciences; is an SAB member of BioInvent and Amgen; and holds a therapeutic Treg cell depletion IP licensed to Takeda.
Figures




Comment in
-
Hair care: Stem cells control immune response during wound repair.Immunity. 2024 May 14;57(5):933-935. doi: 10.1016/j.immuni.2024.04.013. Immunity. 2024. PMID: 38749394
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

