Abnormal changes in metabolites caused by m6A methylation modification: The leading factors that induce the formation of immunosuppressive tumor microenvironment and their promising potential for clinical application
- PMID: 38677545
- PMCID: PMC11976433
- DOI: 10.1016/j.jare.2024.04.016
Abnormal changes in metabolites caused by m6A methylation modification: The leading factors that induce the formation of immunosuppressive tumor microenvironment and their promising potential for clinical application
Abstract
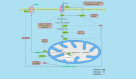
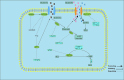
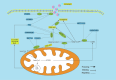
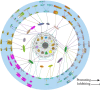
Background: N6-methyladenosine (m6A) RNA methylation modifications have been widely implicated in the metabolic reprogramming of various cell types within the tumor microenvironment (TME) and are essential for meeting the demands of cellular growth and maintaining tissue homeostasis, enabling cells to adapt to the specific conditions of the TME. An increasing number of research studies have focused on the role of m6A modifications in glucose, amino acid and lipid metabolism, revealing their capacity to induce aberrant changes in metabolite levels. These changes may in turn trigger oncogenic signaling pathways, leading to substantial alterations within the TME. Notably, certain metabolites, including lactate, succinate, fumarate, 2-hydroxyglutarate (2-HG), glutamate, glutamine, methionine, S-adenosylmethionine, fatty acids and cholesterol, exhibit pronounced deviations from normal levels. These deviations not only foster tumorigenesis, proliferation and angiogenesis but also give rise to an immunosuppressive TME, thereby facilitating immune evasion by the tumor.
Aim of review: The primary objective of this review is to comprehensively discuss the regulatory role of m6A modifications in the aforementioned metabolites and their potential impact on the development of an immunosuppressive TME through metabolic alterations.
Key scientific concepts of review: This review aims to elaborate on the intricate networks governed by the m6A-metabolite-TME axis and underscores its pivotal role in tumor progression. Furthermore, we delve into the potential implications of the m6A-metabolite-TME axis for the development of novel and targeted therapeutic strategies in cancer research.
Keywords: Cancer; Metabolites; Targeted therapy; Tumor immunosuppressive microenvironment; m(6)A.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Deblois G., Tonekaboni S.A.M., Grillo G., Martinez C., Kao Y.I., Tai F., et al. Epigenetic switch-induced viral mimicry evasion in chemotherapy-resistant breast cancer. Cancer Discov. 2020;10(9):1312–1329. - PubMed
-
- Wang S., Sun C., Li J., Zhang E., Ma Z., Xu W., et al. Roles of RNA methylation by means of N(6)-methyladenosine (m(6)A) in human cancers. Cancer Lett. 2017;408:112–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

