Effects of icosapent ethyl according to baseline residual risk in patients with atherosclerotic cardiovascular disease: results from REDUCE-IT
- PMID: 38678009
- PMCID: PMC11873788
- DOI: 10.1093/ehjcvp/pvae030
Effects of icosapent ethyl according to baseline residual risk in patients with atherosclerotic cardiovascular disease: results from REDUCE-IT
Abstract
Aims: Icosapent ethyl lowers triglycerides and significantly reduces major adverse cardiovascular events (MACE), though treatment effects may vary between individuals. This study aimed to determine the relative and absolute effects of icosapent ethyl on MACE according to baseline cardiovascular disease (CVD) risk in patients with atherosclerotic cardiovascular disease (ASCVD).
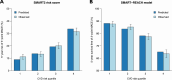
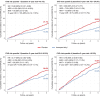
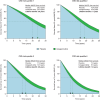
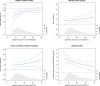
Methods and results: Participants from the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) with ASCVD were included (n = 5785). The primary outcome was 3-point MACE, i.e. non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death. Baseline 5-year risk of MACE was estimated using the European Society of Cardiology (ESC) guideline-recommended SMART2 risk score. Modification of the relative treatment effects of icosapent ethyl by baseline risk was assessed using Cox proportional hazards models, including a treatment-by-risk interaction. Next, treatment effects were assessed stratified by quartiles of baseline risk. During a median follow-up of 4.8 years (interquartile range 3.2-5.3), MACE occurred in 361 vs. 489 patients in the icosapent ethyl vs. placebo group [95% confidence interval (CI)]; hazard ratio (HR) 0.72 (0.63-0.82), absolute risk reduction (ARR) 4.4% (2.6-6.2%), number needed to treat (NNT) 23 (16-38), and 5-year Kaplan-Meier estimated cumulative incidence reduction (CIR) 5.7% (3.5-7.9%). Icosapent ethyl significantly reduced MACE in all risk quartiles, with an HR (95% CI) of 0.62 (0.43-0.88), 0.66 (0.48-0.92), 0.69 (0.53-0.90), and 0.78 (0.63-0.96), respectively (P for treatment-by-risk interaction = 0.106). The ARR (95% CI) increased across risk quartiles, i.e. was 3.9% (1.0-6.8%), 4.3% (1.2-7.3%), 5.1% (1.4-8.7%), and 5.6% (1.3-10.0%), respectively. This translates to NNTs (95% CI) of 26 (15-98), 24 (14-84), 20 (11-70), and 18 (10-77). The 5-year CIR (95% CI) was 4.8% (1.3-8.2%), 5.0% (1.3-8.7%), 6.1% (1.7-10.5%), and 7.7% (2.3-13.2%), respectively. Consistent results were obtained for 5-point MACE, additionally including coronary revascularization and unstable angina.
Conclusion: Among patients with ASCVD and elevated triglyceride levels, icosapent ethyl significantly reduces the risk of MACE irrespective of baseline CVD risk, though absolute benefits are largest for patients at the highest risk.
Keywords: Atherosclerotic cardiovascular disease; Eicosapentaenoic acid; Icosapent ethyl; Residual risk; Secondary prevention; Triglycerides.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PWF, Alberts MJ, D'agostino R, Liau C-S, Mas J-L, Röther J, Smith SC, Salette G, Contant CF, Massaro JM, Steg PG, Reach Registry Investigators FT . Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–1357. 10.1001/jama.2010.1322 - DOI - PubMed
-
- Toth PP, Granowitz C, Hull M, Liassou D, Anderson A, Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc 2018;7:e008740. 10.1161/JAHA.118.008740 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

