Astroglial Kir4.1 potassium channel deficit drives neuronal hyperexcitability and behavioral defects in Fragile X syndrome mouse model
- PMID: 38678030
- PMCID: PMC11055954
- DOI: 10.1038/s41467-024-47681-y
Astroglial Kir4.1 potassium channel deficit drives neuronal hyperexcitability and behavioral defects in Fragile X syndrome mouse model
Abstract
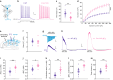
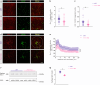
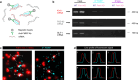
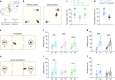
Fragile X syndrome (FXS) is an inherited form of intellectual disability caused by the loss of the mRNA-binding fragile X mental retardation protein (FMRP). FXS is characterized by neuronal hyperexcitability and behavioral defects, however the mechanisms underlying these critical dysfunctions remain unclear. Here, using male Fmr1 knockout mouse model of FXS, we identify abnormal extracellular potassium homeostasis, along with impaired potassium channel Kir4.1 expression and function in astrocytes. Further, we reveal that Kir4.1 mRNA is a binding target of FMRP. Finally, we show that the deficit in astroglial Kir4.1 underlies neuronal hyperexcitability and several behavioral defects in Fmr1 knockout mice. Viral delivery of Kir4.1 channels specifically to hippocampal astrocytes from Fmr1 knockout mice indeed rescues normal astrocyte potassium uptake, neuronal excitability, and cognitive and social performance. Our findings uncover an important role for astrocyte dysfunction in the pathophysiology of FXS, and identify Kir4.1 channel as a potential therapeutic target for FXS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bakker C, et al. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 683154/EC | EC Seventh Framework Programm | FP7 Ideas: European Research Council (FP7-IDEAS-ERC - Specific Programme: "Ideas" Implementing the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007 to 2013))
- 722053/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 1535/Fondation Jérôme Lejeune (Jérôme Lejeune Foundation)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

