Computational modeling reveals key factors driving treatment-free remission in chronic myeloid leukemia patients
- PMID: 38678088
- PMCID: PMC11055880
- DOI: 10.1038/s41540-024-00370-4
Computational modeling reveals key factors driving treatment-free remission in chronic myeloid leukemia patients
Abstract
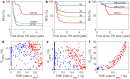
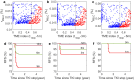
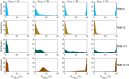
Patients with chronic myeloid leukemia (CML) who receive tyrosine kinase inhibitors (TKIs) have been known to achieve treatment-free remission (TFR) upon discontinuing treatment. However, the underlying mechanisms of this phenomenon remain incompletely understood. This study aims to elucidate the mechanism of TFR in CML patients, focusing on the feedback interaction between leukemia stem cells and the bone marrow microenvironment. We have developed a mathematical model to explore the interplay between leukemia stem cells and the bone marrow microenvironment, allowing for the simulation of CML progression dynamics. Our proposed model reveals a dichotomous response following TKI discontinuation, with two distinct patient groups emerging: one prone to early molecular relapse and the other capable of achieving long-term TFR after treatment cessation. This finding aligns with clinical observations and underscores the essential role of feedback interaction between leukemic cells and the tumor microenvironment in sustaining TFR. Notably, we have shown that the ratio of leukemia cells in peripheral blood (PBLC) and the tumor microenvironment (TME) index can be a valuable predictive tool for identifying patients likely to achieve TFR after discontinuing treatment. This study provides fresh insights into the mechanism of TFR in CML patients and underscores the significance of microenvironmental control in achieving TFR.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
[Research Progress on Tyrosine Kinase Inhibitors Discontinuation in Patients with Chronic Myeloid Leukemia --Review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2025 Feb;33(1):300-305. doi: 10.19746/j.cnki.issn.1009-2137.2025.01.046. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2025. PMID: 40017224 Review. Chinese.
-
Low-dose tyrosine kinase inhibitors before treatment discontinuation do not impair treatment-free remission in chronic myeloid leukemia patients: Results of a retrospective study.Cancer. 2020 Aug 1;126(15):3438-3447. doi: 10.1002/cncr.32940. Epub 2020 May 27. Cancer. 2020. PMID: 32459375
-
Leukemic stem cells shall be searched in the bone marrow before "tyrosine kinase inhibitor-discontinuation" in chronic myeloid leukemia.Int J Lab Hematol. 2021 Oct;43(5):1110-1116. doi: 10.1111/ijlh.13528. Epub 2021 Apr 9. Int J Lab Hematol. 2021. PMID: 33834631
-
The e13a2 BCR-ABL transcript negatively affects sustained deep molecular response and the achievement of treatment-free remission in patients with chronic myeloid leukemia who receive tyrosine kinase inhibitors.Cancer. 2019 May 15;125(10):1674-1682. doi: 10.1002/cncr.31977. Epub 2019 Feb 1. Cancer. 2019. PMID: 30707758
-
[Tyrosine kinase inhibitor therapy discontinuation for chronic myelogenous leukemia to achieve clinical cure: current status and future perspectives].Rinsho Ketsueki. 2018;59(10):2094-2103. doi: 10.11406/rinketsu.59.2094. Rinsho Ketsueki. 2018. PMID: 30305514 Review. Japanese.
References
Publication types
MeSH terms
Substances
Grants and funding
- 11831015/National Natural Science Foundation of China (National Science Foundation of China)
- 12331018/National Natural Science Foundation of China (National Science Foundation of China)
- 12171478/National Science Foundation of China | National Natural Science Foundation of China-Yunnan Joint Fund (NSFC-Yunnan Joint Fund)
LinkOut - more resources
Full Text Sources
Medical

