Effectiveness of DialBetesPlus, a self-management support system for diabetic kidney disease: Randomized controlled trial
- PMID: 38678094
- PMCID: PMC11055918
- DOI: 10.1038/s41746-024-01114-8
Effectiveness of DialBetesPlus, a self-management support system for diabetic kidney disease: Randomized controlled trial
Erratum in
-
Author Correction: Effectiveness of DialBetesPlus, a self-management support system for diabetic kidney disease: Randomized controlled trial.NPJ Digit Med. 2024 Jun 15;7(1):155. doi: 10.1038/s41746-024-01158-w. NPJ Digit Med. 2024. PMID: 38879663 Free PMC article. No abstract available.
Abstract
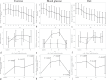
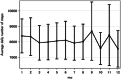
We evaluated the effectiveness of a mobile health (mHealth) intervention for diabetic kidney disease patients by conducting a 12-month randomized controlled trial among 126 type 2 diabetes mellitus patients with moderately increased albuminuria (urinary albumin-to-creatinine ratio (UACR): 30-299 mg/g creatinine) recruited from eight clinical sites in Japan. Using a Theory of Planned Behavior (TPB) behavior change theory framework, the intervention provides patients detailed information in order to improve patient control over exercise and dietary behaviors. In addition to standard care, the intervention group received DialBetesPlus, a self-management support system allowing patients to monitor exercise, blood glucose, diet, blood pressure, and body weight via a smartphone application. The primary outcome, change in UACR after 12 months (used as a surrogate measure of renal function), was 28.8% better than the control group's change (P = 0.029). Secondary outcomes also improved in the intervention group, including a 0.32-point better change in HbA1c percentage (P = 0.041). These improvements persisted when models were adjusted to account for the impacts of coadministration of drugs targeting albuminuria (GLP-1 receptor agonists, SGLT-2 inhibitors, ACE inhibitors, and ARBs) (UACR: -32.3% [95% CI: -49.2%, -9.8%] between-group difference in change, P = 0.008). Exploratory multivariate regression analysis suggests that the improvements were primarily due to levels of exercise. This is the first trial to show that a lifestyle intervention via mHealth achieved a clinically-significant improvement in moderately increased albuminuria.
© 2024. The Author(s).
Conflict of interest statement
K.W. has received research sponsorship/grants from NTT DOCOMO Inc. and Nihon Chouzai Co, Ltd.; and consulted for Nihon Chouzai Co, Ltd. During the study, K.W., A.S., and K.M. were members of a cooperative program between the University of Tokyo and NTT DOCOMO. The remaining authors declare no competing interests.
Figures



References
-
- Magliano D. J., Boyko E. J. IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS [Internet]. 10th ed. Brussels: International Diabetes Federation; 2021.
-
- Tofte N, et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8:301–312. doi: 10.1016/S2213-8587(20)30026-7. - DOI - PubMed
Grants and funding
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
- JP19ek0210095/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Miscellaneous

