Sphingosine-1-phosphate receptor 1/5 selective agonist alleviates ocular vascular pathologies
- PMID: 38678148
- PMCID: PMC11055896
- DOI: 10.1038/s41598-024-60540-6
Sphingosine-1-phosphate receptor 1/5 selective agonist alleviates ocular vascular pathologies
Abstract
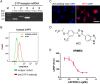
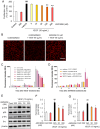
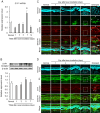
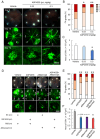
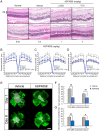
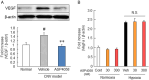
Ocular abnormal angiogenesis and edema are featured in several ocular diseases. S1P signaling via S1P1 likely is part of the negative feedback mechanism necessary to maintain vascular health. In this study, we conducted pharmacological experiments to determine whether ASP4058, a sphingosine 1-phosphate receptor 1/5 (S1P1/5) agonist, is useful in abnormal vascular pathology in the eye. First, human retinal microvascular endothelial cells (HRMECs) were examined using vascular endothelial growth factor (VEGF)-induced cell proliferation and hyperpermeability. ASP4058 showed high affinity and inhibited VEGF-induced proliferation and hyperpermeability of HRMECs. Furthermore, S1P1 expression and localization changes were examined in the murine laser-induced choroidal neovascularization (CNV) model, a mouse model of exudative age-related macular degeneration, and the efficacy of ASP4058 was verified. In the CNV model mice, S1P1 tended to decrease in expression immediately after laser irradiation and colocalized with endothelial cells and Müller glial cells. Oral administration of ASP4058 also suppressed vascular hyperpermeability and CNV, and the effect was comparable to that of the intravitreal administration of aflibercept, an anti-VEGF drug. Next, efficacy was also examined in a retinal vein occlusion (RVO) model in which retinal vascular permeability was increased. ASP4058 dose-dependently suppressed the intraretinal edema. In addition, it suppressed the expansion of the perfusion area observed in the RVO model. ASP4058 also suppressed the production of VEGF in the eye. Collectively, ASP4058 can be a potential therapeutic agent that normalizes abnormal vascular pathology, such as age-related macular degeneration and RVO, through its direct action on endothelial cells.
© 2024. The Author(s).
Conflict of interest statement
S.N., M.S., S.N., and H.H. received a research grant from Astellas Pharma Inc. R.Y., T.M., and S.K. are employees of Astellas Pharma Inc. Y.I. and M.G. are employees of Astellas Institute for Regenerative Medicine. H.Y., A.N., and K.T. declare no competing interests.
Figures
Similar articles
-
ApoM-bound S1P acts via endothelial S1PR1 to suppress choroidal neovascularization and vascular leakage.Angiogenesis. 2025 Apr 23;28(2):24. doi: 10.1007/s10456-025-09975-7. Angiogenesis. 2025. PMID: 40266369 Free PMC article.
-
A sphingosine-1-phosphate receptor type 1 agonist, ASP4058, suppresses intracranial aneurysm through promoting endothelial integrity and blocking macrophage transmigration.Br J Pharmacol. 2017 Jul;174(13):2085-2101. doi: 10.1111/bph.13820. Epub 2017 May 27. Br J Pharmacol. 2017. PMID: 28409823 Free PMC article.
-
Apatinib, an Inhibitor of Vascular Endothelial Growth Factor Receptor 2, Suppresses Pathologic Ocular Neovascularization in Mice.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3592-3599. doi: 10.1167/iovs.17-21416. Invest Ophthalmol Vis Sci. 2017. PMID: 28715845
-
Nrf2 Activator RS9 Suppresses Pathological Ocular Angiogenesis and Hyperpermeability.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):1943-1952. doi: 10.1167/iovs.18-25745. Invest Ophthalmol Vis Sci. 2019. PMID: 31050722
-
Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis.BMJ Open. 2019 May 28;9(5):e022031. doi: 10.1136/bmjopen-2018-022031. BMJ Open. 2019. PMID: 31142516 Free PMC article.
Cited by
-
ApoM-bound S1P acts via endothelial S1PR1 to suppress choroidal neovascularization and vascular leakage.Angiogenesis. 2025 Apr 23;28(2):24. doi: 10.1007/s10456-025-09975-7. Angiogenesis. 2025. PMID: 40266369 Free PMC article.
-
miRNA-6236 Regulation of Postischemic Skeletal Muscle Angiogenesis.J Am Heart Assoc. 2024 Dec 3;13(23):e035923. doi: 10.1161/JAHA.124.035923. Epub 2024 Nov 27. J Am Heart Assoc. 2024. PMID: 39604034 Free PMC article.
References
-
- Nguyen DH, Luo J, Zhang K, Zhang M. Current therapeutic approaches in neovascular age-related macular degeneration. Discov. Med. 2013;15:343–348. - PubMed
-
- Maguire MG, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–1761. doi: 10.1016/j.ophtha.2016.03.045. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

