Economic evaluations of medical devices in paediatrics: a systematic review and a quality appraisal of the literature
- PMID: 38678250
- PMCID: PMC11056067
- DOI: 10.1186/s12962-024-00537-0
Economic evaluations of medical devices in paediatrics: a systematic review and a quality appraisal of the literature
Abstract
Background: Although economic evaluations (EEs) have been increasingly applied to medical devices, little discussion has been conducted on how the different health realities of specific populations may impact the application of methods and the ensuing results. This is particularly relevant for pediatric populations, as most EEs on devices are conducted in adults, with specific aspects related to the uniqueness of child health often being overlooked. This study provides a review of the published EEs on devices used in paediatrics, assessing the quality of reporting, and summarising methodological challenges.
Methods: A systematic literature search was performed to identify peer-reviewed publications on the economic value of devices used in paediatrics in the form of full EEs (comparing both costs and consequences of two or more devices). After the removal of duplicates, article titles and abstracts were screened. The remaining full-text articles were retrieved and assessed for inclusion. In-vitro diagnostic devices were not considered in this review. Study descriptive and methodological characteristics were extracted using a structured template. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 checklist was used to assess the quality of reporting. A narrative synthesis of the results was conducted followed by a critical discussion on the main challenges found in the literature.
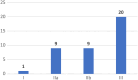
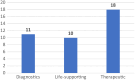
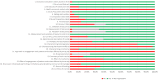
Results: 39 full EEs were eligible for review. Most studies were conducted in high-income countries (67%) and focused on high-risk therapeutic devices (72%). Studies comprised 25 cost-utility analyses, 13 cost-effectiveness analyses and 1 cost-benefit analysis. Most of the studies considered a lifetime horizon (41%) and a health system perspective (36%). Compliance with the CHEERS 2022 items varied among the studies.
Conclusions: Despite the scant body of evidence on EEs focusing on devices in paediatrics results highlight the need to improve the quality of reporting and advance methods that can explicitly incorporate the multiple impacts related to the use of devices with distinct characteristics, as well as consider specific child health realities. The design of innovative participatory approaches and instruments for measuring outcomes meaningful to children and their families should be sought in future research.
Keywords: Cost-effectiveness; Economic evaluation; Health technology assessment; Medical devices; Pediatrics; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- van den Heuvel R, Kapadia A, Stirling C, Zhou J. Medical devices 2030. KPMG Int. 2018.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources