Perampanel for Treatment of People with a Range of Epilepsy Aetiologies in Clinical Practice: Evidence from the PERMIT Extension Study
- PMID: 38678505
- PMCID: PMC11136933
- DOI: 10.1007/s40120-024-00618-5
Perampanel for Treatment of People with a Range of Epilepsy Aetiologies in Clinical Practice: Evidence from the PERMIT Extension Study
Abstract
Introduction: It is important to assess the effectiveness of an antiseizure medication in treating different epilepsy aetiologies to optimise individualised therapeutic approaches. Data from the PERaMpanel pooled analysIs of effecTiveness and tolerability (PERMIT) Extension study were used to assess the effectiveness and safety/tolerability of perampanel (PER) when used to treat individuals with a range of epilepsy aetiologies in clinical practice.
Methods: A post hoc analysis was conducted of PERMIT Extension data from individuals with a known aetiology. Retention was assessed after 3, 6 and 12 months. Effectiveness was assessed after 3, 6 and 12 months and at the last visit (last observation carried forward). Effectiveness assessments included responder rate (≥ 50% seizure frequency reduction) and seizure freedom rate (no seizures since at least the prior visit). Safety/tolerability was assessed by evaluating adverse events (AEs) and AEs leading to discontinuation.
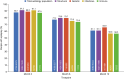
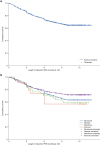
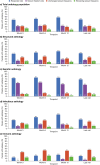
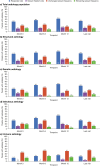
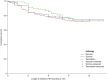
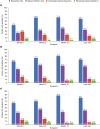
Results: PERMIT Extension included 1945 individuals with structural aetiology, 1012 with genetic aetiology, 93 with an infectious aetiology, and 26 with an immune aetiology. Retention rates at 12 months were 61.1% (structural), 65.9% (genetic), 56.8% (infectious) and 56.5% (immune). At the last visit, responder rates (total seizures) were 43.3% (structural), 68.3% (genetic), 37.0% (infectious) and 20.0% (immune), and corresponding seizure freedom rates were 15.8%, 46.5%, 11.1% and 5.0%, respectively. AE incidence rates were 58.0% (structural), 46.5% (genetic), 51.1% (infectious) and 65.0% (immune), and corresponding rates of discontinuation due to AEs over 12 months were 18.9%, 16.4%, 18.5% and 21.7%, respectively. The types of AEs reported were generally consistent across aetiology subgroups, with no idiosyncratic AEs emerging.
Conclusion: Although PER was effective and generally well tolerated when used to treat individuals with a range of epilepsy aetiologies in clinical practice, variability in its effectiveness and tolerability across the subgroups indicates that PER may be particularly useful for individuals with specific epilepsy aetiologies.
Keywords: Anticonvulsant; Antiepileptic drug; Antiseizure medication; Focal seizures; Generalized seizures; Real-world.
© 2024. The Author(s).
Conflict of interest statement
Adam Strzelczyk reports personal fees and grants from Angelini Pharma, Bicodex, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Precisis, Takeda, UCB Pharma, and UNEEG medical. Marta Maschio has no conflict of interest. Max C. Pensel has no conflict of interest. Antonietta Coppola has received speaker fees from Eisai and consultancy fees from GW Pharmaceuticals/Jazz Pharmaceuticals, UCB and Bial-Portela & Cª. Satoru Takahashi has no conflict of interest. Shuichi Izumoto has no conflict of interest. Eugen Trinka reports personal fees from EVER Pharma, Marinus, Arvelle, Angelini, Argenx, Medtronic, Bial-Portela & Cª, NewBridge, GL Pharma, GlaxoSmithKline, Boehringer Ingelheim, LivaNova, Eisai, UCB, Biogen, Sanofi, Jazz Pharmaceuticals, and Actavis. His institution received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Österreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubiläumsfond der Österreichischen Nationalbank. Sheri Cappucci is an employee of Eisai. Ricardo Sainz-Fuertes is an employee of Eisai. Vicente Villanueva has participated in advisory boards and symposia organised by Angelini, Bial, Biocodex, Eisai Inc, Jazz Pharmaceuticals, Novartis, Takeda, UCB and Xenon.
Figures

References
LinkOut - more resources
Full Text Sources

