Smooth muscle cell-derived Cxcl12 directs macrophage accrual and sympathetic innervation to control thermogenic adipose tissue
- PMID: 38678562
- PMCID: PMC11413973
- DOI: 10.1016/j.celrep.2024.114169
Smooth muscle cell-derived Cxcl12 directs macrophage accrual and sympathetic innervation to control thermogenic adipose tissue
Abstract
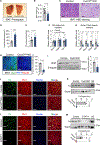
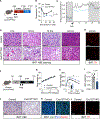
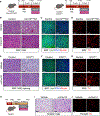
Sympathetic innervation of brown adipose tissue (BAT) controls mammalian adaptative thermogenesis. However, the cellular and molecular underpinnings contributing to BAT innervation remain poorly defined. Here, we show that smooth muscle cells (SMCs) support BAT growth, lipid utilization, and thermogenic plasticity. Moreover, we find that BAT SMCs express and control the bioavailability of Cxcl12. SMC deletion of Cxcl12 fosters brown adipocyte lipid accumulation, reduces energy expenditure, and increases susceptibility to diet-induced metabolic dysfunction. Mechanistically, we find that Cxcl12 stimulates CD301+ macrophage recruitment and supports sympathetic neuronal maintenance. Administering recombinant Cxcl12 to obese mice or leptin-deficient (Ob/Ob) mice is sufficient to boost macrophage presence and drive sympathetic innervation to restore BAT morphology and thermogenic responses. Altogether, our data reveal an SMC chemokine-dependent pathway linking immunological infiltration and sympathetic innervation as a rheostat for BAT maintenance and thermogenesis.
Keywords: CP: Immunology; CP: Metabolism; Cxcl2; brown adipocytes; macrophage; smooth muscle cells; sympathetic innervation; thermogenesis.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

