Establishment of Swine Primary Nasal, Tracheal, and Bronchial Epithelial Cell Culture Models for the Study of Influenza Virus Infection
- PMID: 38679164
- PMCID: PMC11129919
- DOI: 10.1016/j.jviromet.2024.114943
Establishment of Swine Primary Nasal, Tracheal, and Bronchial Epithelial Cell Culture Models for the Study of Influenza Virus Infection
Abstract
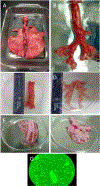
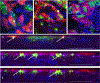
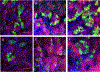
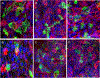
We established primary porcine nasal, tracheal, and bronchial epithelial cells that recapitulate the physical and functional properties of the respiratory tract and have the ability to fully differentiate. Trans-well cultures demonstrated increased transepithelial electrical resistance over time the presence of tight junctions as demonstrated by immunohistochemistry. The nasal, tracheal, and bronchial epithelial cells developed cilia, secreted mucus, and expressed sialic acids on surface glycoproteins, the latter which are required for influenza A virus infection. Swine influenza viruses were shown to replicate efficiently in the primary epithelial cell cultures, supporting the use of these culture models to assess swine influenza and other virus infection. Primary porcine nasal, tracheal, and bronchial epithelial cell culture models enable assessment of emerging and novel influenza viruses for pandemic potential as well as mechanistic studies to understand mechanisms of infection, reassortment, and generation of novel virus. As swine are susceptible to infection with multiple viral and bacterial respiratory pathogens, these primary airway cell models may enable study of the cellular response to infection by pathogens associated with Porcine Respiratory Disease Complex.
Keywords: Epithelial cells; Influenza A virus; Models; N-Acetylneuraminic acid; Swine.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
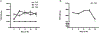
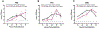
Similar articles
-
The Originally Established PBE Cell Line as a Reliable In Vitro Model for Investigating SIV Infection and Immunity.Int J Mol Sci. 2025 Jun 16;26(12):5764. doi: 10.3390/ijms26125764. Int J Mol Sci. 2025. PMID: 40565228 Free PMC article.
-
Long-term culture of chicken tracheal organoids for the purpose of avian influenza virus research.Virol J. 2025 Apr 15;22(1):99. doi: 10.1186/s12985-025-02714-w. Virol J. 2025. PMID: 40234888 Free PMC article.
-
A MicroRNA Screen Identifies the Wnt Signaling Pathway as a Regulator of the Interferon Response during Flavivirus Infection.J Virol. 2017 Mar 29;91(8):e02388-16. doi: 10.1128/JVI.02388-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148804 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
Cited by
-
The Mucus-Binding Factor Mediates Lacticaseibacillus rhamnosus CRL1505 Adhesion but Not Immunomodulation in the Respiratory Tract.Microorganisms. 2024 Jun 16;12(6):1209. doi: 10.3390/microorganisms12061209. Microorganisms. 2024. PMID: 38930591 Free PMC article.
-
Pandemic Risk Assessment for Swine Influenza A Virus in Comparative In Vitro and In Vivo Models.Viruses. 2024 Mar 31;16(4):548. doi: 10.3390/v16040548. Viruses. 2024. PMID: 38675891 Free PMC article.
-
The Originally Established PBE Cell Line as a Reliable In Vitro Model for Investigating SIV Infection and Immunity.Int J Mol Sci. 2025 Jun 16;26(12):5764. doi: 10.3390/ijms26125764. Int J Mol Sci. 2025. PMID: 40565228 Free PMC article.
-
Porcine Airway Organoid-Derived Well-Differentiated Epithelial Cultures as a Tool for the Characterization of Swine Influenza a Virus Strains.Viruses. 2024 Nov 15;16(11):1777. doi: 10.3390/v16111777. Viruses. 2024. PMID: 39599891 Free PMC article.
-
Interplay between respiratory viruses and cilia in the airways.Eur Respir Rev. 2025 Mar 19;34(175):240224. doi: 10.1183/16000617.0224-2024. Print 2025 Jan. Eur Respir Rev. 2025. PMID: 40107662 Free PMC article. Review.
References
-
- Brockmeier SL, Halbur PG, Thacker EL, 2002. Porcine Respiratory Disease Complex Chapter 13. Polymicrobial Diseases.
-
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR and Randell SH, 2005. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107, 183–206. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

