Does perioperative hydrocortisone or indomethacin improve pancreatoduodenectomy outcomes? A triple arm, randomized placebo-controlled trial
- PMID: 38679455
- PMCID: PMC11341883
- DOI: 10.14701/ahbps.24-021
Does perioperative hydrocortisone or indomethacin improve pancreatoduodenectomy outcomes? A triple arm, randomized placebo-controlled trial
Abstract
Backgrounds/aims: This trial evaluated whether anti-inflammatory agents hydrocortisone (H) and indomethacin (I) could reduce major complications after pancreatoduodenectomy (PD).
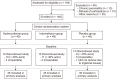
Methods: Between June 2018 and June 2020, 105 patients undergoing PD with > 40% of acini on the intraoperative frozen section were randomized into three groups (35 patients per group): 1) intravenous H 100 mg 8 hourly, 2) rectal I suppository 100 mg 12 hourly, and 3) placebo (P) from postoperative day (POD) 0-2. Participants, investigators, and outcome assessors were blinded. The primary outcome was major complications (Clavien-Dindo grades 3-5). Secondary outcomes were overall complications (Clavien-Dindo grades 1-5), Clinically relevant postoperative pancreatic fistula (CR-POPF), delayed gastric emptying (DGE), postpancreatectomy hemorrhage (PPH), surgical site infections (SSI), length of stay, POD-3 serum amylase, readmission rate, and mortality.
Results: Major complications were comparable (8.6%, 5.7%, and 8.6% in groups H, I, and P, respectively). However, overall complications were significantly lower in group H than in group P (45.7% vs. 80.0%, p = 0.006). CR-POPF (14.3% vs. 25.7%, p = 0.371), PPH (8.6% vs. 14.3%, p = 0.710), DGE (8.6% vs. 22.9%, p = 0.188), and SSI (14.3% vs. 25.7%, p = 0.371) were comparable between groups H and P. Major complications and overall complications in group I were 5.7% and 60.0%, respectively, which were comparable to those in groups P and H. CR-POPF rates in groups H, I, and P were 14.3%, 17.1%, and 25.7%, respectively, which was comparable.
Conclusions: H and I did not decrease major complications in PD.
Keywords: Complications; Hydrocortisone; Indomethacin; Pancreatic fistula; Pancreatoduodenectomy.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
LinkOut - more resources
Full Text Sources