A positive feedback inhibition of isocitrate dehydrogenase 3β on paired-box gene 6 promotes Alzheimer-like pathology
- PMID: 38679634
- PMCID: PMC11056379
- DOI: 10.1038/s41392-024-01812-5
A positive feedback inhibition of isocitrate dehydrogenase 3β on paired-box gene 6 promotes Alzheimer-like pathology
Erratum in
-
Correction: A positive feedback inhibition of isocitrate dehydrogenase 3β on paired-box gene 6 promotes Alzheimer-like pathology.Signal Transduct Target Ther. 2024 Jul 15;9(1):187. doi: 10.1038/s41392-024-01904-2. Signal Transduct Target Ther. 2024. PMID: 39009590 Free PMC article. No abstract available.
Abstract
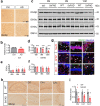
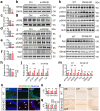
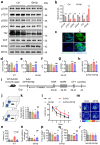
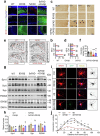
Impaired brain glucose metabolism is an early indicator of Alzheimer's disease (AD); however, the fundamental mechanism is unknown. In this study, we found a substantial decline in isocitrate dehydrogenase 3β (IDH3β) levels, a critical tricarboxylic acid cycle enzyme, in AD patients and AD-transgenic mice's brains. Further investigations demonstrated that the knockdown of IDH3β induced oxidation-phosphorylation uncoupling, leading to reduced energy metabolism and lactate accumulation. The resulting increased lactate, a source of lactyl, was found to promote histone lactylation, thereby enhancing the expression of paired-box gene 6 (PAX6). As an inhibitory transcription factor of IDH3β, the elevated PAX6 in turn inhibited the expression of IDH3β, leading to tau hyperphosphorylation, synapse impairment, and learning and memory deficits resembling those seen in AD. In AD-transgenic mice, upregulating IDH3β and downregulating PAX6 were found to improve cognitive functioning and reverse AD-like pathologies. Collectively, our data suggest that impaired oxidative phosphorylation accelerates AD progression via a positive feedback inhibition loop of IDH3β-lactate-PAX6-IDH3β. Breaking this loop by upregulating IDH3β or downregulating PAX6 attenuates AD neurodegeneration and cognitive impairments.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- 82230041/National Natural Science Foundation of China (National Science Foundation of China)
- 91949205/National Natural Science Foundation of China (National Science Foundation of China)
- 31730035/National Natural Science Foundation of China (National Science Foundation of China)
- 81721005/National Natural Science Foundation of China (National Science Foundation of China)
- 82301624/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

