Mesenchymal stromal/stem cells promote intestinal epithelium regeneration after chemotherapy-induced damage
- PMID: 38679715
- PMCID: PMC11057078
- DOI: 10.1186/s13287-024-03738-9
Mesenchymal stromal/stem cells promote intestinal epithelium regeneration after chemotherapy-induced damage
Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative treatment for leukemia and a range of non-malignant disorders. The success of the therapy is hampered by occurrence of acute graft-versus-host disease (aGvHD); an inflammatory response damaging recipient organs, with gut, liver, and skin being the most susceptible. Intestinal GvHD injury is often a life-threatening complication in patients unresponsive to steroid treatment. Allogeneic mesenchymal stromal/stem cell (MSC) infusions are a promising potential treatment for steroid-resistant aGvHD. Data from our institution and others demonstrate rescue of approximately 40-50% of aGvHD patients with MSCs in Phase I, II studies and minor side effects. Although promising, better understanding of MSC mode of action and patient response to MSC-based therapy is essential to improve this lifesaving treatment.
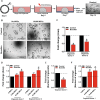
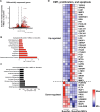
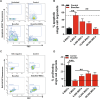
Methods: Single cell human small intestine organoids were embedded in Matrigel, grown for 5 days and treated with busulfan for 48 h. Organoids damaged by treatment with busulfan or control organoids were co-cultured with 5000, 10,000, and 50,000 MSCs for 24 h, 48 h or 7 days and the analyses such as surface area determination, proliferation and apoptosis assessment, RNA sequencing and proteomics were performed.
Results: Here, we developed a 3D co-culture model of human small intestinal organoids and MSCs, which allows to study the regenerative effects of MSCs on intestinal epithelium in a more physiologically relevant setting than existing in vitro systems. Using this model we mimicked chemotherapy-mediated damage of the intestinal epithelium. The treatment with busulfan, the chemotherapeutic commonly used as conditioning regiment before the HSCT, affected pathways regulating epithelial to mesenchymal transition, proliferation, and apoptosis in small intestinal organoids, as shown by transcriptomic and proteomic analysis. The co-culture of busulfan-treated intestinal organoids with MSCs reversed the effects of busulfan on the transcriptome and proteome of intestinal epithelium, which we also confirmed by functional evaluation of proliferation and apoptosis.
Conclusions: Collectively, we demonstrate that our in vitro co-culture system is a new valuable tool to facilitate the investigation of the molecular mechanisms behind the therapeutic effects of MSCs on damaged intestinal epithelium. This could benefit further optimization of the use of MSCs in HSCT patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Salvage treatment of steroid-refractory acute GVHD with the off-the-shelf product of human umbilical cord mesenchymal stromal cells: a multicenter, open label, phase Ib/IIa trial.Stem Cell Res Ther. 2025 Jul 1;16(1):345. doi: 10.1186/s13287-025-04446-8. Stem Cell Res Ther. 2025. PMID: 40597363 Free PMC article. Clinical Trial.
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2024 Nov 7;11(11):CD010189. doi: 10.1002/14651858.CD010189.pub3. Cochrane Database Syst Rev. 2024. PMID: 39508306
-
Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis.Lancet Haematol. 2016 Jan;3(1):e45-52. doi: 10.1016/S2352-3026(15)00224-0. Epub 2015 Nov 27. Lancet Haematol. 2016. PMID: 26765648
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009159. doi: 10.1002/14651858.CD009159.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2023 Jun 21;6:CD009159. doi: 10.1002/14651858.CD009159.pub3. PMID: 22972135 Updated.
Cited by
-
Three-Dimensional Bioprinting and Infertility-Related Female Reproductive System Diseases: A Review of Current and Future Applications.Tissue Eng Regen Med. 2025 Aug 19. doi: 10.1007/s13770-025-00754-5. Online ahead of print. Tissue Eng Regen Med. 2025. PMID: 40828232 Review.
-
Network-Based Bioinformatics Highlights Broad Importance of Human Milk Hyaluronan.Int J Mol Sci. 2024 Nov 26;25(23):12679. doi: 10.3390/ijms252312679. Int J Mol Sci. 2024. PMID: 39684390 Free PMC article.
-
Advances and applications of gut organoids: modeling intestinal diseases and therapeutic development.Life Med. 2025 Mar 7;4(2):lnaf012. doi: 10.1093/lifemedi/lnaf012. eCollection 2025 Apr. Life Med. 2025. PMID: 40276096 Free PMC article. Review.
-
Salvage treatment of steroid-refractory acute GVHD with the off-the-shelf product of human umbilical cord mesenchymal stromal cells: a multicenter, open label, phase Ib/IIa trial.Stem Cell Res Ther. 2025 Jul 1;16(1):345. doi: 10.1186/s13287-025-04446-8. Stem Cell Res Ther. 2025. PMID: 40597363 Free PMC article. Clinical Trial.
-
The therapeutic potential of mesenchymal stem cells in intestinal diseases: from mechanisms to clinical translation.Stem Cell Res Ther. 2025 Jul 21;16(1):393. doi: 10.1186/s13287-025-04523-y. Stem Cell Res Ther. 2025. PMID: 40691643 Free PMC article. Review.
References
-
- Jansen SA, et al. Chemotherapy-induced intestinal injury promotes Galectin-9-driven modulation of T cell function. bioRxiv. 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

