Plasma ALS and Gal-3BP differentiate early from advanced liver fibrosis in MASLD patients
- PMID: 38679739
- PMCID: PMC11057169
- DOI: 10.1186/s40364-024-00583-z
Plasma ALS and Gal-3BP differentiate early from advanced liver fibrosis in MASLD patients
Abstract
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) is estimated to affect 30% of the world's population, and its prevalence is increasing in line with obesity. Liver fibrosis is closely related to mortality, making it the most important clinical parameter for MASLD. It is currently assessed by liver biopsy - an invasive procedure that has some limitations. There is thus an urgent need for a reliable non-invasive means to diagnose earlier MASLD stages.
Methods: A discovery study was performed on 158 plasma samples from histologically-characterised MASLD patients using mass spectrometry (MS)-based quantitative proteomics. Differentially abundant proteins were selected for verification by ELISA in the same cohort. They were subsequently validated in an independent MASLD cohort (n = 200).
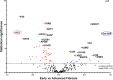
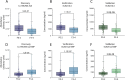
Results: From the 72 proteins differentially abundant between patients with early (F0-2) and advanced fibrosis (F3-4), we selected Insulin-like growth factor-binding protein complex acid labile subunit (ALS) and Galectin-3-binding protein (Gal-3BP) for further study. In our validation cohort, AUROCs with 95% CIs of 0.744 [0.673 - 0.816] and 0.735 [0.661 - 0.81] were obtained for ALS and Gal-3BP, respectively. Combining ALS and Gal-3BP improved the assessment of advanced liver fibrosis, giving an AUROC of 0.796 [0.731. 0.862]. The {ALS; Gal-3BP} model surpassed classic fibrosis panels in predicting advanced liver fibrosis.
Conclusions: Further investigations with complementary cohorts will be needed to confirm the usefulness of ALS and Gal-3BP individually and in combination with other biomarkers for diagnosis of liver fibrosis. With the availability of ELISA assays, these findings could be rapidly clinically translated, providing direct benefits for patients.
Keywords: Biomarker; Fibrosis; Liver; MASLD; Proteomics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Rinella ME, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966. - PubMed
Grants and funding
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-10-INBS-08]/ProFI project
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-17-EURE-0003]/Chemistry Biology Health (CBH) Graduate School at University Grenoble Alpes
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-15-IDEX-02]/LIFE project
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
- [ANR-19-P3IA-0003]/MIAI @ Grenoble Alpes
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

