Precise capture of circulating endometrial cells in endometriosis
- PMID: 38679794
- PMCID: PMC11268826
- DOI: 10.1097/CM9.0000000000002910
Precise capture of circulating endometrial cells in endometriosis
Abstract
Background: Endometriosis (EM) is a complex benign gynecological disease, but it has malignant biological behavior and can invade any part of the body. Clinical manifestations include pelvic pain, dysmenorrhea, infertility, pelvic nodules, and masses. Our previous study successfully detected circulating endometrial cells (CECs) in the peripheral blood of patients with EM. The purpose of this study is to overcome the limitation of cell size in the previous microfluidic chip method, to further accurately capture CECs, understand the characteristics of these cells, and explore the relationship between CECs and the clinical course characteristics of patients with EM.
Methods: Human peripheral venous blood used to detect CECs and circulating vascular endothelial cells (CVECs) was taken from EM patients ( n = 34) hospitalized in the Peking University People's Hospital. We used the subtraction enrichment and immunostaining fluorescence in situ hybridization (SE-iFISH) method to exclude the interference of red blood cells, white blood cells, and CVECs, so as to accurately capture the CECs in the peripheral blood of patients with EM. Then we clarified the size and ploidy number of chromosome 8 of CECs, and a second grouping of patients was performed based on clinical characteristics to determine the relationship between CECs and clinical course characteristics.
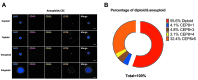
Results: The peripheral blood of 34 EM patients and 12 non-EM patients was evaluated by SE-iFISH. Overall, 34 eligible EM patients were enrolled. The results showed that the detection rates of CECs were 58.8% in EM patients and 16.7% in the control group. However, after classification according to clinical characteristics, more CECs could be detected in the peripheral blood of patients with rapidly progressive EM, with a detection rate of 94.4% (17/18). In total, 63.5% (40/63) of these cells were small cells with diameters below 5 μm, and 44.4% (28/63) were aneuploid cells. No significant association was found between the number of CECs and EM stage.
Conclusion: The number and characteristics of CECs are related to the clinical course characteristics of patients with EM, such as pain and changes in lesion size, and may be used as biomarkers for personalized treatment and management of EM in the future.
Copyright © 2024 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures


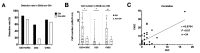


Similar articles
-
Evaluation of Circulating Endometrial Cells as a Biomarker for Endometriosis.Chin Med J (Engl). 2017 Oct 5;130(19):2339-2345. doi: 10.4103/0366-6999.215325. Chin Med J (Engl). 2017. PMID: 28937041 Free PMC article.
-
Circulating Endometrial Cells in Women With Spontaneous Pneumothorax.Chest. 2020 Feb;157(2):342-355. doi: 10.1016/j.chest.2019.09.008. Epub 2019 Sep 19. Chest. 2020. PMID: 31542450
-
Circulating endometrial cells in peripheral blood.Eur J Obstet Gynecol Reprod Biol. 2014 Oct;181:267-74. doi: 10.1016/j.ejogrb.2014.07.037. Epub 2014 Aug 20. Eur J Obstet Gynecol Reprod Biol. 2014. PMID: 25195200
-
Aneuploid CTC and CEC.Diagnostics (Basel). 2018 Apr 18;8(2):26. doi: 10.3390/diagnostics8020026. Diagnostics (Basel). 2018. PMID: 29670052 Free PMC article. Review.
-
Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis.Med Sci Monit. 2011 Apr;17(4):RA92-9. doi: 10.12659/msm.881707. Med Sci Monit. 2011. PMID: 21455119 Free PMC article. Review.
Cited by
-
Precision Therapeutic and Preventive Molecular Strategies for Endometriosis-Associated Infertility.Int J Mol Sci. 2025 Aug 9;26(16):7706. doi: 10.3390/ijms26167706. Int J Mol Sci. 2025. PMID: 40869026 Free PMC article. Review.
-
Endometriosis is not the endometrium: Reviewing the over-representation of eutopic endometrium in endometriosis research.Elife. 2025 May 20;14:e103825. doi: 10.7554/eLife.103825. Elife. 2025. PMID: 40392231 Free PMC article. Review.
References
-
- Sampson JA. Perforating hemorrhagic (chocolate) cysts of the ovary. Their importance and especially their relation to pelvic adenomas of the endometrial type. Arch Surg 1921;3: 245–323. doi: 10.1001/archsurg.1921.01110080003001.
-
- Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med 2020;382: 1244–1256. doi: 10.1056/NEJMra1810764. - PubMed
-
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers 2018;4: 9. doi: 10.1038/s41572-018-0008-5. - PubMed
-
- Leyland N, Casper R, Laberge P, Singh SS. Sogc. Endometriosis: Diagnosis and management. J Obstet Gynaecol Can 2010;32: S1–S32. doi: 10.1016/S1701-2163(16)34589-3. - PubMed
-
- Yang B, Wang T, Li N, Zhang W, Hu Y. The high expression of RRM2 can predict the malignant transformation of endometriosis. Adv Ther 2021;38: 5178–5190. doi: 10.1007/s12325-021-01888-3. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

