Tuning Electron-Accepting Properties of Phthalocyanines for Charge Transfer Processes
- PMID: 38679903
- PMCID: PMC11094797
- DOI: 10.1021/acs.inorgchem.4c00527
Tuning Electron-Accepting Properties of Phthalocyanines for Charge Transfer Processes
Abstract
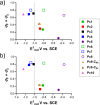
Phthalocyanines play fundamental roles as electron-acceptors in many different fields; thus, the study of structural features affecting electron-accepting properties of these macrocycles is highly desirable. A series of low-symmetry zinc(II) phthalocyanines, in which one, three, or four benzene rings were replaced for pyrazines, was prepared and decorated with electron-neutral (alkylsulfanyl) or strongly electron-withdrawing (alkylsulfonyl) groups to study the role of the macrocyclic core as well as the effect of peripheral substituents. Electrochemical studies revealed that the first reduction potential (Ered1) is directly proportional to the number of pyrazine units in the macrocycle. Introduction of alkylsulfonyl groups had a very strong effect and resulted in a strongly electron-deficient macrocycle with Ered1 = -0.48 V vs SCE (in THF). The efficiency of intramolecular-charge transfer (ICT) from the peripheral bis(2-methoxyethyl)amine group to the macrocycle was monitored as a decrease in the sum of ΦΔ + ΦF and correlated well with the determined Ered1 values. The strongest quenching by ICT was observed for the most electron-deficient macrocycle. Importantly, an obvious threshold at -1.0 V vs SCE was observed over which no ICT occurs. Disclosed results may substantially help to improve the design of electron-donor systems based on phthalocyanines.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Tam L. K. B.; Yu L. G.; Wong R. C. H.; Fong W. P.; Ng D. K. P.; Lo P. C. Dual Cathepsin B and Glutathione-Activated Dimeric and Trimeric Phthalocyanine-Based Photodynamic Molecular Beacons for Targeted Photodynamic Therapy. J. Med. Chem. 2021, 64 (23), 17455–17467. 10.1021/acs.jmedchem.1c01634. - DOI - PubMed
-
- Chen S.-Y.; Li Z.; Li K.; Yu X.-Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 21369110.1016/j.ccr.2020.213691. - DOI
-
- Urbani M.; Ragoussi M. E.; Nazeeruddin M. K.; Torres T. Phthalocyanines for dye-sensitized solar cells. Coord. Chem. Rev. 2019, 381, 1–64. 10.1016/j.ccr.2018.10.007. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

