Treatment with nanosomal paclitaxel lipid suspension versus conventional paclitaxel in metastatic breast cancer patients - a multicenter, randomized, comparative, phase II/III clinical study
- PMID: 38680290
- PMCID: PMC11047258
- DOI: 10.1177/17588359241236442
Treatment with nanosomal paclitaxel lipid suspension versus conventional paclitaxel in metastatic breast cancer patients - a multicenter, randomized, comparative, phase II/III clinical study
Abstract
Background: A novel nanosomal paclitaxel lipid suspension (NPLS), free from Cremophor EL (CrEL) and ethanol, was developed to address the solvent-related toxicities associated with conventional paclitaxel formulation.
Objective: To evaluate the efficacy and safety of NPLS versus CrEL-based paclitaxel (conventional paclitaxel) in patients with metastatic breast cancer (MBC).
Design: A prospective, open-label, randomized, multiple-dose, parallel, phase II/III study.
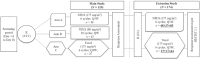
Methods: Adult (18-65 years) female patients with MBC who had previously failed at least one line of chemotherapy were randomized (2:2:1) to NPLS 175 mg/m2 every 3 weeks (Q3W, n = 48, arm A), NPLS 80 mg/m2 every week (QW, n = 45, arm B) without premedication or conventional paclitaxel (Taxol®, manufactured by Bristol-Myers Squibb, Princeton, NJ, USA) 175 mg/m2 Q3W (n = 27, arm C) with premedication. In the extension study, an additional 54 patients were randomized (2:1) to arm A (n = 37) or arm C (n = 17).
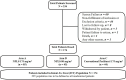
Results: Pooled data from the primary study and its extension phase included 174 patients. The primary endpoint was the overall response rate (ORR). As per intent-to-treat analysis, ORR was significantly better in the NPLS QW arm as compared to conventional paclitaxel [44.4% (20/45) versus 22.7% (10/44), (p = 0.04)]. An improvement in ORR with NPLS Q3W versus conventional paclitaxel arm [29.4% (25/85) versus 22.7% (10/44)] (p = 0.53) was observed. Disease control rates observed were improved with NPLS Q3W versus conventional paclitaxel Q3W (77.7% versus 72.7%, p = 0.66) and with NPLS QW versus conventional paclitaxel Q3W (84.4% versus 72.7%, p = 0.20), although not significant. A lower incidence of grade III/IV peripheral sensory neuropathy, vomiting, and dyspnea was reported with NPLS Q3W versus conventional paclitaxel Q3W arms.
Conclusion: NPLS demonstrated an improved tumor response rate and a favorable safety profile versus conventional paclitaxel. NPLS 80 mg/m2 QW demonstrated a significantly better response versus conventional paclitaxel 175 mg/m2 Q3W.
Trial registration: Clinical Trial Registry-India (CTRI), CTRI/2010/091/001344 Registered on: 18 October 2010 (https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=MjEzNQ==&Enc=&userName=CTRI/2010/091/001344), CTRI/2015/07/006062 Registered on: 31 July 2015 (https://ctri.nic.in/Clinicaltrials/pmaindet2.php?EncHid=MTE2Mjc=&Enc=&userName=CTRI/2015/07/006062).
Keywords: NPLS; PacliAqualip; breast cancer; metastatic; nanosomal paclitaxel lipid suspension; paclitaxel.
Plain language summary
Role of nanosomal paclitaxel lipid suspension (NPLS) in the treatment of patients with metastatic breast cancer (MBC) Why was the study done? Paclitaxel is a commonly used drug for the treatment of breast cancer. Conventional formulation of paclitaxel is known to cause side effects like injection site reactions. A newer formulation named NPLS was developed to overcome the limitations of the conventional paclitaxel. The current study was done to compare the safety and effectiveness of NPLS and conventional paclitaxel in patients with advanced breast cancer. What did the researchers do? The research team conducted a large study in multiple hospitals across India, involving women with advanced breast cancer who had experienced treatment failure with previous chemotherapy. A total of 174 patients were randomly assigned to receive either of the three treatment schedules: (1) NPLS every 3 weeks, (2) NPLS every week, (3) conventional paclitaxel every 3 weeks. What did the researchers find? The results showed that NPLS, in a weekly schedule, led to better tumor response rates compared to conventional paclitaxel given every 3 weeks. Additionally, NPLS demonstrated a favorable safety profile, as compared to conventional paclitaxel. What do the findings mean? These findings suggest that NPLS could be a promising alternative for women with advanced breast cancer. NPLS improved the response to treatment, with a better safety profile compared to conventional paclitaxel.
© The Author(s), 2024.
Conflict of interest statement
Dr Ateeq Ahmad, Dr Saifuddin Sheikh, Dr Shoukath M Ali, and Dr Imran Ahmad are employees of Jina Pharmaceuticals, Libertyville, IL, USA. Mr Ronak Patel is an employee of Lambda Therapeutic Research Limited, Ahmedabad India. Mr Mahesh Paithankar, Dr Lav Patel, Dr Anil Rajani, Dr Deepak Bunger, and Dr Alok Chaturvedi are employees of Intas Pharmaceuticals Limited, Ahmedabad, India.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al.. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- National Institute for Cancer Prevention and Research. GLOBOCAN 2020: India factsheet, https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-she... (2020, accessed 25 April 2022).
-
- Malvia S, Bagadi SA, Dubey US, et al.. Epidemiology of breast cancer in Indian women. Asia-Pac J Clin Oncol 2017; 13: 289–295. - PubMed
-
- Anampa J, Sparano JA. New agents for the management of resistant metastatic breast cancer. Expert Opin Pharmacother 2017; 18: 1815–1831. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

