MNDA, a PYHIN factor involved in transcriptional regulation and apoptosis control in leukocytes
- PMID: 38680493
- PMCID: PMC11045911
- DOI: 10.3389/fimmu.2024.1395035
MNDA, a PYHIN factor involved in transcriptional regulation and apoptosis control in leukocytes
Abstract
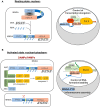
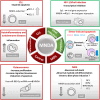
Inflammation control is critical during the innate immune response. Such response is triggered by the detection of molecules originating from pathogens or damaged host cells by pattern-recognition receptors (PRRs). PRRs subsequently initiate intra-cellular signalling through different pathways, resulting in i) the production of inflammatory cytokines, including type I interferon (IFN), and ii) the initiation of a cascade of events that promote both immediate host responses as well as adaptive immune responses. All human PYRIN and HIN-200 domains (PYHIN) protein family members were initially proposed to be PRRs, although this view has been challenged by reports that revealed their impact on other cellular mechanisms. Of relevance here, the human PYHIN factor myeloid nuclear differentiation antigen (MNDA) has recently been shown to directly control the transcription of genes encoding factors that regulate programmed cell death and inflammation. While MNDA is mainly found in the nucleus of leukocytes of both myeloid (neutrophils and monocytes) and lymphoid (B-cell) origin, its subcellular localization has been shown to be modulated in response to genotoxic agents that induce apoptosis and by bacterial constituents, mediators of inflammation. Prior studies have noted the importance of MNDA as a marker for certain forms of lymphoma, and as a clinical prognostic factor for hematopoietic diseases characterized by defective regulation of apoptosis. Abnormal expression of MNDA has also been associated with altered levels of cytokines and other inflammatory mediators. Refining our comprehension of the regulatory mechanisms governing the expression of MNDA and other PYHIN proteins, as well as enhancing our definition of their molecular functions, could significantly influence the management and treatment strategies of numerous human diseases. Here, we review the current state of knowledge regarding PYHIN proteins and their role in innate and adaptive immune responses. Emphasis will be placed on the regulation, function, and relevance of MNDA expression in the control of gene transcription and RNA stability during cell death and inflammation.
Keywords: MNDA; PYHIN factors; apoptosis; genotoxic stress; innate immunity; transcription control.
Copyright © 2024 Bottardi, Layne, Ramòn, Quansah, Wurtele, Affar and Milot.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

