Novel insights into immune-related genes associated with type 2 diabetes mellitus-related cognitive impairment
- PMID: 38680704
- PMCID: PMC11045412
- DOI: 10.4239/wjd.v15.i4.735
Novel insights into immune-related genes associated with type 2 diabetes mellitus-related cognitive impairment
Abstract
Background: The cognitive impairment in type 2 diabetes mellitus (T2DM) is a multifaceted and advancing state that requires further exploration to fully comprehend. Neuroinflammation is considered to be one of the main mechanisms and the immune system has played a vital role in the progression of the disease.
Aim: To identify and validate the immune-related genes in the hippocampus associated with T2DM-related cognitive impairment.
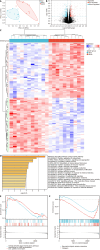
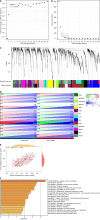
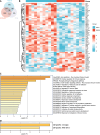
Methods: To identify differentially expressed genes (DEGs) between T2DM and controls, we used data from the Gene Expression Omnibus database GSE125387. To identify T2DM module genes, we used Weighted Gene Co-Expression Network Analysis. All the genes were subject to Gene Set Enrichment Analysis. Protein-protein interaction network construction and machine learning were utilized to identify three hub genes. Immune cell infiltration analysis was performed. The three hub genes were validated in GSE152539 via receiver operating characteristic curve analysis. Validation experiments including reverse transcription quantitative real-time PCR, Western blotting and immunohistochemistry were conducted both in vivo and in vitro. To identify potential drugs associated with hub genes, we used the Comparative Toxicogenomics Database (CTD).
Results: A total of 576 DEGs were identified using GSE125387. By taking the intersection of DEGs, T2DM module genes, and immune-related genes, a total of 59 genes associated with the immune system were identified. Afterward, machine learning was utilized to identify three hub genes (H2-T24, Rac3, and Tfrc). The hub genes were associated with a variety of immune cells. The three hub genes were validated in GSE152539. Validation experiments were conducted at the mRNA and protein levels both in vivo and in vitro, consistent with the bioinformatics analysis. Additionally, 11 potential drugs associated with RAC3 and TFRC were identified based on the CTD.
Conclusion: Immune-related genes that differ in expression in the hippocampus are closely linked to microglia. We validated the expression of three hub genes both in vivo and in vitro, consistent with our bioinformatics results. We discovered 11 compounds associated with RAC3 and TFRC. These findings suggest that they are co-regulatory molecules of immunometabolism in diabetic cognitive impairment.
Keywords: Bioinformatics analysis; Cognitive impairment; Hippocampus; Immune; Microglia; Type 2 diabetes mellitus.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors assert that there are no conflicting interests.
Figures

Similar articles
-
Screening and identification of potential hub genes and immune cell infiltration in the synovial tissue of rheumatoid arthritis by bioinformatic approach.Heliyon. 2023 Jan 10;9(1):e12799. doi: 10.1016/j.heliyon.2023.e12799. eCollection 2023 Jan. Heliyon. 2023. PMID: 36699262 Free PMC article.
-
Identification of Key Genes and Pathways in the Hippocampus after Traumatic Brain Injury: Bioinformatics Analysis and Experimental Validation.J Integr Neurosci. 2023 Feb 20;22(2):44. doi: 10.31083/j.jin2202044. J Integr Neurosci. 2023. PMID: 36992583
-
Shared peripheral blood biomarkers for Alzheimer's disease, major depressive disorder, and type 2 diabetes and cognitive risk factor analysis.Heliyon. 2023 Mar 17;9(3):e14653. doi: 10.1016/j.heliyon.2023.e14653. eCollection 2023 Mar. Heliyon. 2023. PMID: 36994393 Free PMC article.
-
Identification of Effective Diagnostic Biomarkers and Immune Cell Infiltration in Atopic Dermatitis by Comprehensive Bioinformatics Analysis.Front Mol Biosci. 2022 Jul 14;9:917077. doi: 10.3389/fmolb.2022.917077. eCollection 2022. Front Mol Biosci. 2022. PMID: 35911963 Free PMC article.
-
Identification and analysis of hub genes of hypoxia-immunity in type 2 diabetes mellitus.Front Genet. 2023 Apr 21;14:1154839. doi: 10.3389/fgene.2023.1154839. eCollection 2023. Front Genet. 2023. PMID: 37153000 Free PMC article.
References
-
- Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8:535–545. - PubMed
-
- Huang C, Luo J, Wen X, Li K. Linking Diabetes Mellitus with Alzheimer's Disease: Bioinformatics Analysis for the Potential Pathways and Characteristic Genes. Biochem Genet. 2022;60:1049–1075. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

