Pathways for Diagnosing and Treating CKD-Associated Pruritus: A Narrative Review
- PMID: 38680970
- PMCID: PMC11047256
- DOI: 10.1177/20543581241238808
Pathways for Diagnosing and Treating CKD-Associated Pruritus: A Narrative Review
Abstract
Purpose of review: Chronic kidney disease (CKD)-associated pruritus is a common, persistent, and distressing itch experienced by patients across the CKD spectrum. Although the disorder is associated with adverse outcomes and poor health-related quality of life, it remains underdiagnosed and undertreated. The purpose of this narrative review is to offer health care providers guidance on how to effectively identify, assess, and treat patients with CKD-associated pruritus, with the goal of reducing symptom burden and improving patient-important outcomes, such as quality of life (QoL).
Sources of information: A panel of nephrologists and researchers from across Canada and the United States was assembled to develop this narrative review based on the best available data, current treatment guidelines, and their clinical experiences.
Methods: A panel of nephrologists who actively care for patients with pruritus receiving dialysis from across Canada was assembled. Two researchers from the United States were also included based on their expertise in the diagnosis and management of CKD-associated pruritus. Throughout Spring 2023, the panel met to discuss key topics in the identification, assessment, and management of CKD-associated pruritus. Panel members subsequently developed summaries of the pertinent information based on the best available data, current treatment guidelines, and added information on their own clinical experiences. In all cases, approval of the article was sought and achieved through discussion.
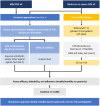
Key findings: This narrative review provides pragmatic guidance addressing: (1) methods for screening CKD-associated pruritus, (2) assessing severity, (3) management of CKD-associated pruritus, and (4) suggested areas for future research. The panel developed a 3-pillar framework for proactive assessment and severity scoring in CKD-aP: systematic screening for CKD-associated pruritus (pillar 1), assessment of pruritus intensity (pillar 2), and understanding the impact of CKD-associated pruritus on the patient's QoL (pillar 3). Management of CKD-associated pruritus can include ensuring optimization of dialysis adequacy, achieving mineral metabolism targets (ie, calcium, phosphate, and parathyroid hormone). However, treatment of CKD-associated pruritus usually requires additional interventions. Patients, regardless of CKD-associated pruritus severity, should be counseled on adequate skin hydration and other non-pharmacological strategies to reduce pruritus. Antihistamines should be avoided in favor of evidence-based treatments, such as difelikefalin and gabapentin.
Limitations: A formal systematic review (SR) of the literature was not undertaken, although published SRs were reviewed. The possibility for bias based on the experts' own clinical experiences may have occurred. Key takeaways are based on the current available evidence, of which head-to-head clinical trials are lacking.
Funding: This work was funded by an arm's length grant from Otsuka Canada Pharmaceutical Inc. (the importer and distributer of difelikefalin in Canada). LiV Medical Education Agency Inc. provided logistical and editorial support.
Motif de la revue: Le prurit associé à l’insuffisance rénale chronique (IRC) est une démangeaison cutanée fréquente, persistante et invalidante que les patients de tout le specter de l’IRC peuvent ressentir. Bien que le prurit soit associé à des effets indésirables et à une mauvaise qualité de vie liée à la santé, il demeure sous-diagnostiqué et sous-traité. L’objectif de cette revue narrative est d’offrir des conseils aux professionnels de la santé sur la façon d’identifier, d’évaluer et de traiter efficacement les patients atteints de prurit associé à l’IRC; ceci dans le but de réduire la charge des symptômes et d’améliorer les résultats importants pour les patients, notamment leur qualité de vie (QdV).
Sources de l’information: Un comité de néphrologues et de chercheurs de partout au Canada et des États-Unis a été constitué pour élaborer la présente revue narrative à partir des meilleures données disponibles, des lignes directrices actuelles pour le traitement et de leurs expériences cliniques.
Méthodologie: Un groupe de néphrologues canadiens qui s’occupent activement de patients dialysés souffrant de prurit a été constitué. Deux chercheurs des États-Unis ont été inclus au groupe en raison de leur expertise dans le diagnostic et la prise en charge du prurit associé à l’IRC. Le comité s’est réuni tout au long du printemps 2023 pour discuter de sujets clés en lien avec l’identification, l’évaluation et la prise en charge du prurit associé à l’IRC. Les membres du comité ont par la suite rédigé des résumés des informations pertinentes en se basant sur les meilleures données disponibles et les lignes directrices actuelles pour le traitement, auxquels ils ont ajouté des informations issues de leurs propres expériences cliniques. Dans tous les cas, l’approbation du manuscrit a été sollicitée et obtenue par discussion.
Principaux résultats: Cette revue narrative offre des conseils pragmatiques sur les points suivants: (1) les méthodes de dépistage du prurit associé à l’IRC; (2) l’évaluation de sa gravité; (3) sa prise en charge; et (4) les domaines suggérés pour de futures recherches. Le comité a développé un cadre à trois piliers pour l’évaluation proactive du prurit associé à l’IRC et l’établissement d’un score de gravité: le dépistage systématique du prurit associé à l’IRC (pilier 1), l’évaluation de son intensité (pilier 2) et la compréhension de son impact sur la QdV du patient (pilier 3). La prise en charge du prurit associé à l’IRC peut inclure l’optimisation de l’adéquation de la dialyse et l’atteinte des cibles du métabolisme minéral (c.-à-d. calcium, phosphate et hormone parathyroïdienne). Cependant, son traitement nécessite habituellement des interventions supplémentaires. Les patients, quelle que soit la gravité du prurit associé à l’IRC, devraient être avisés d’hydrater adéquatement leur peau et informés des autres stratégies non pharmacologiques afin de réduire le prurit. On devrait éviter les antihistaminiques et les remplacer par des traitements fondés sur des données probantes comme la difélikéfaline et la gabapentine.
Limites: Aucune revue systématique de la littérature n’a été formellement entreprise, bien que les revues systématiques publiées aient été examinées. La possibilité d’un biais fondé sur les expériences cliniques des experts est envisageable. Les principales conclusions de cette étude sont fondées sur les données probantes actuellement disponibles, pour lesquelles il n’existe pas d’essais cliniques comparatifs.
Financement: Ces travaux ont été financés par une subvention indépendante d’Otsuka Canada Pharmaceutical Inc. (l’importateur et distributeur de la difélikéfaline au Canada). Un soutien logistique et éditorial a été fourni par liV Medical Education Agency Inc.
Keywords: CKD-associated pruritus treatment; assessment; chronic kidney disease-associated pruritus; dialysis; screening.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Claudio Rigatto: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Boehringer Ingelheim, Astra Zeneca, Sanofi; Grants/Clinical trials: Sanofi. David Collister: Speaker’s bureau/honoraria: N/A; Grants/investigator: Canadian Institutes of Health Research: RESET-DIALYSIS, Canadian Institutes of Health Research: GAHT-KIDNEY, Kidney Foundation of Canada: RESET-DIALYSIS, KRESCENT post-doctoral fellowship, KRESCENT new investigator award, Research Manitoba/Boehringer Ingelheim: Virtual Kidney Check and Follow-Up; I am national leader for POSIBIL-6 which is sponsored by CSL-Behring but fees are directed to my research program. Alexandre Granger-Vallée: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, GSK. Louis Girard: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Alexion, Bayer, BI-Lilly, AstraZeneca, Janssen, Merck, Bausch Health, CPD Network, and Sanofi; Grants/investigator: Chemocentryx, Otsuka (the importer and distributer of difelikefalin in Canada) and Visterra. Jay Hingwala: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, GSK; Grants/investigator: Otsuka (the importer and distributer of difelikefalin in Canada). Angelo Karaboyas: Dr Karaboyas is an employee of Arbor Research Collaborative for Health, which administers the DOPPS. Global support for the ongoing DOPPS Program is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All funds are made to Arbor Research Collaborative for Health and not directly to Dr Karaboyas. Adeera Levin: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, AZ, Gilead, GSK, Jansen; Grants/Clinical trials: KFOC/CIHR, Jansen, BI, AZ, GSK. Philip McFarlane: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Alexion, AMGEN, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Janssen, Lilly, Sanofi-Aventis, and Vifor; Grants/investigator: Otsuka (the importer and distributer of difelikefalin in Canada), Alexion, AstraZeneca, Bayer, Boehringer Ingelheim, Fresenius, GSK, Janssen, Novartis. Ron Pisoni: Dr Pisoni is an employee of Arbor Research Collaborative for Health, which administers the DOPPS. Global support for the ongoing DOPPS Program is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All funds are made to Arbor Research Collaborative for Health and not directly to Dr Pisoni. Bhanu Prasad: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer; Grants/Clinical trials: Medtronic. Normand Proulx: Speaker’s bureau/honoraria: AMGEN, AstraZeneca, Bayer, Beigene, BMS, Eli Lilly, EMD Serono, Ipsen, Merck, Novartis, Pfizer, Roche, Sanofi, Servier, Takeda. Daniel Schwartz: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, Janssen, BI, Lilly; Grants/investigator: CPD Network, Endocrine Research Society. Manish Sood: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), AstraZeneca, Bayer, and GlaxoSmithKline. Rita Suri: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, Astra Zeneca, GSK, Amgen. Karthik Tennankore: Speaker’s bureau/honoraria: Otsuka (the importer and distributer of difelikefalin in Canada), Bayer, Baxter, GSK, Vifor Pharmaceuticals, Virtual Hallway; Grants/investigator: Multiple, but industry specific grant: unrestricted grant funding for an investigator-initiated project on CKD-aP severity measurement algorithm in hemodialysis.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

