Comparative transcriptomic analysis reveals the molecular changes of acute pancreatitis in experimental models
- PMID: 38681131
- PMCID: PMC11045495
- DOI: 10.3748/wjg.v30.i14.2038
Comparative transcriptomic analysis reveals the molecular changes of acute pancreatitis in experimental models
Abstract
Background: Acute pancreatitis (AP) encompasses a spectrum of pancreatic inflammatory conditions, ranging from mild inflammation to severe pancreatic necrosis and multisystem organ failure. Given the challenges associated with obtaining human pancreatic samples, research on AP predominantly relies on animal models. In this study, we aimed to elucidate the fundamental molecular mechanisms underlying AP using various AP models.
Aim: To investigate the shared molecular changes underlying the development of AP across varying severity levels.
Methods: AP was induced in animal models through treatment with caerulein alone or in combination with lipopolysaccharide (LPS). Additionally, using Ptf1α to drive the specific expression of the hM3 promoter in pancreatic acinar cells transgenic C57BL/6J- hM3/Ptf1α(cre) mice were administered Clozapine N-oxide to induce AP. Subsequently, we conducted RNA sequencing of pancreatic tissues and validated the expression of significantly different genes using the Gene Expression Omnibus (GEO) database.
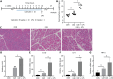
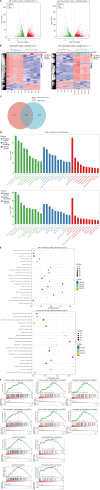
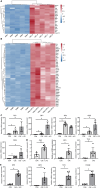
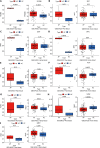
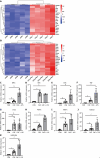
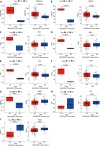
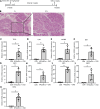
Results: Caerulein-induced AP showed severe inflammation and edema, which were exacerbated when combined with LPS and accompanied by partial pancreatic tissue necrosis. Compared with the control group, RNA sequencing analysis revealed 880 significantly differentially expressed genes in the caerulein model and 885 in the caerulein combined with the LPS model. Kyoto Encyclopedia of Genes and Genomes enrichment analysis and Gene Set Enrichment Analysis indicated substantial enrichment of the TLR and NOD-like receptor signaling pathway, TLR signaling pathway, and NF-κB signaling pathway, alongside elevated levels of apoptosis-related pathways, such as apoptosis, P53 pathway, and phagosome pathway. The significantly elevated genes in the TLR and NOD-like receptor signaling pathways, as well as in the apoptosis pathway, were validated through quantitative real-time PCR experiments in animal models. Validation from the GEO database revealed that only MYD88 concurred in both mouse pancreatic tissue and human AP peripheral blood, while TLR1, TLR7, RIPK3, and OAS2 genes exhibited marked elevation in human AP. The genes TUBA1A and GADD45A played significant roles in apoptosis within human AP. The transgenic mouse model hM3/Ptf1α(cre) successfully validated significant differential genes in the TLR and NOD-like receptor signaling pathways as well as the apoptosis pathway, indicating that these pathways represent shared pathological processes in AP across different models.
Conclusion: The TLR and NOD receptor signaling pathways play crucial roles in the inflammatory progression of AP, notably the MYD88 gene. Apoptosis holds a central position in the necrotic processes of AP, with TUBA1A and GADD45A genes exhibiting prominence in human AP.
Keywords: Acute pancreatitis; Apoptosis; Experimental acute pancreatitis models; Inflammatory; RNA-sequencing; TLR and NOD-like signaling pathways.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no competing interests.
Figures

References
-
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726–734. - PubMed
-
- Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. - PubMed
-
- Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–1193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

