Exploring nature's antidote: unveiling the inhibitory potential of selected medicinal plants from Kisumu, Kenya against venom from some snakes of medical significance in sub-Saharan Africa
- PMID: 38681195
- PMCID: PMC11045943
- DOI: 10.3389/fphar.2024.1369768
Exploring nature's antidote: unveiling the inhibitory potential of selected medicinal plants from Kisumu, Kenya against venom from some snakes of medical significance in sub-Saharan Africa
Abstract
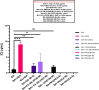
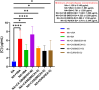
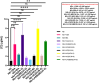
Background: The present study investigated the efficacy of Conyza bonariensis, Commiphora africana, Senna obtusifolia, Warburgia ugandensis, Vernonia glabra, and Zanthoxylum usambarense against Bitis arietans venom (BAV), Naja ashei venom (NAV), and Naja subfulva venom (NSV). Methods: 40 extracts and fractions were prepared using n-hexane, dichloromethane, ethyl acetate, and methanol. In vitro efficacy against snake venom phospholipase A2 (svPLA2) was determined in 96-well microtiter and agarose-egg yolk coagulation assays. in vivo efficacy against venom-induced cytotoxicity was determined using Artemia salina. Two commercial antivenoms were used for comparison. Results: The 96-well microtiter assay revealed poor svPLA2 inhibition of BAV by antivenom (range: 20.76% ± 13.29% to 51.29% ± 3.26%) but strong inhibition (>90%) by dichloromethane and hexane fractions of C. africana, hexane and ethyl acetate extracts and fraction of W. ugandensis, dichloromethane fraction of V. glabra, and the methanol extract of S. obtusifolia. The methanol extract and fraction of C. africana, and the hexane extract of Z. usambarense strongly inhibited (>90%) svPLA2 activity in NAV. The hexane and ethyl acetate fractions of V. glabra and the dichloromethane, ethyl acetate, and methanol extracts of C. africana strongly inhibited (>90%) svPLA2 in NSV. The agarose egg yolk coagulation assay showed significant inhibition of BAV by the dichloromethane fraction of C. africana (EC50 = 3.51 ± 2.58 μg/mL), significant inhibition of NAV by the methanol fraction of C. africana (EC50 = 7.35 ± 1.800 μg/mL), and significant inhibition of NSV by the hexane extract of V. glabra (EC50 = 7.94 ± 1.50 μg/mL). All antivenoms were non-cytotoxic in A. salina but the methanol extract of C. africana and the hexane extracts of V. glabra and Z. usambarense were cytotoxic. The dichloromethane fraction of C. africana significantly neutralized BAV-induced cytotoxicity, the methanol fraction and extract of C. africana neutralized NAV-induced cytotoxicity, while the ethyl acetate extract of V. glabra significantly neutralized NSV-induced cytotoxicity. Glycosides, flavonoids, phenolics, and tannins were identified in the non-cytotoxic extracts/fractions. Conclusion: These findings validate the local use of C. africana and V. glabra in snakebite but not C. bonariensis, S. obtusifolia, W. ugandensis, and Z. usambarense. Further work is needed to isolate pure compounds from the effective plants and identify their mechanisms of action.
Keywords: Artemia salina bioassay; Bitis arietans; Naja ashei; Naja subfulva; medicinal plants; preclinical efficacy evaluation; snake venom.
Copyright © 2024 Okumu, Mbaria, Gikunju, Mbuthia, Madadi and Ochola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Enzymatic activity and brine shrimp lethality of venom from the large brown spitting cobra (Naja ashei) and its neutralization by antivenom.BMC Res Notes. 2020 Jul 6;13(1):325. doi: 10.1186/s13104-020-05167-2. BMC Res Notes. 2020. PMID: 32631407 Free PMC article.
-
Artemia salina as an animal model for the preliminary evaluation of snake venom-induced toxicity.Toxicon X. 2021 Aug 19;12:100082. doi: 10.1016/j.toxcx.2021.100082. eCollection 2021 Nov. Toxicon X. 2021. PMID: 34471870 Free PMC article.
-
In Vitro and In Vivo Neutralizing Activity of Uvaria chamae Leaves Fractions on the Venom of Naja nigricollis in Albino Rat and Bovine Blood.Recent Pat Biotechnol. 2020;14(4):295-311. doi: 10.2174/1872208314666200903152129. Recent Pat Biotechnol. 2020. PMID: 32885765
-
Phytochemical characterization and phospholipase A2 inhibitory effect of Vitex negundo L. root extracts.J Ethnopharmacol. 2024 Apr 6;323:117671. doi: 10.1016/j.jep.2023.117671. Epub 2023 Dec 30. J Ethnopharmacol. 2024. PMID: 38163555
-
Unveiling the potential of medicinal herbs as the source for in vitro screening toward the inhibition of Nrf2.Heliyon. 2024 Sep 26;10(19):e38411. doi: 10.1016/j.heliyon.2024.e38411. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39416811 Free PMC article. Review.
References
-
- Abdullahi H. L., Muhammed H. S., Tajuddeen N., Hamza S. A., Ibrahim M. A. (2017). Effect of methanolicstem-bark extract of Commiphora pedunculata on plasma recalcification time of Najani gricollis venom treated plasma. Bayero J. Pure Appl. Sci. 10, 233–237. 10.4314/bajopas.v10i2.38 - DOI
-
- Amadi B. A., Agomuo E. N., Ibegbulem C. O. (2004). Proximate analysis. Res. Methods Biochem. Supreme Publ. Owerri, Niger., 105–115.
-
- Atanassova M., Georgieva S., Ivancheva K. (2011). Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. J. Univ. Chem. Technol. Metall. 46.
LinkOut - more resources
Full Text Sources

