Temporal allele frequency changes in large-effect loci reveal potential fishing impacts on salmon life-history diversity
- PMID: 38681510
- PMCID: PMC11046039
- DOI: 10.1111/eva.13690
Temporal allele frequency changes in large-effect loci reveal potential fishing impacts on salmon life-history diversity
Abstract
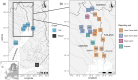
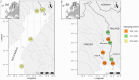
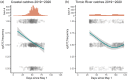
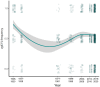
Fishing has the potential to influence the life-history traits of exploited populations. However, our understanding of how fisheries can induce evolutionary genetic changes remains incomplete. The discovery of large-effect loci linked with ecologically important life-history traits, such as age at maturity in Atlantic salmon (Salmo salar), provides an opportunity to study the impacts of temporally varying fishing pressures on these traits. A 93-year archive of fish scales from wild Atlantic salmon catches from the northern Baltic Sea region allowed us to monitor variation in adaptive genetic diversity linked with age at maturity of wild Atlantic salmon populations. The dataset consisted of samples from both commercial and recreational fisheries that target salmon on their spawning migration. Using a genotyping-by-sequencing approach (GT-seq), we discovered strong within-season allele frequency changes at the vgll3 locus linked with Atlantic salmon age at maturity: fishing in the early season preferentially targeted the vgll3 variant linked with older maturation. We also found within-season temporal variation in catch proportions of different wild Atlantic salmon subpopulations. Therefore, selective pressures of harvesting may vary depending on the seasonal timing of fishing, which has the potential to cause evolutionary changes in key life-history traits and their diversity. This knowledge can be used to guide fisheries management to reduce the effects of fishing practices on salmon life-history diversity. Thus, this study provides a tangible example of using genomic approaches to infer, monitor and help mitigate human impacts on adaptively important genetic variation in nature.
Keywords: Baltic salmon; SNP; fisheries management; fisheries‐induced evolution; genetic stock identification; temporal genomics.
© 2024 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Co-inheritance of sea age at maturity and iteroparity in the Atlantic salmon vgll3 genomic region.J Evol Biol. 2019 Apr;32(4):343-355. doi: 10.1111/jeb.13418. Epub 2019 Feb 22. J Evol Biol. 2019. PMID: 30697850
-
Rapid evolution in salmon life history induced by direct and indirect effects of fishing.Science. 2022 Apr 22;376(6591):420-423. doi: 10.1126/science.abg5980. Epub 2022 Feb 24. Science. 2022. PMID: 35201899
-
Population and size-specific distribution of Atlantic salmon Salmo salar in the Baltic Sea over five decades.J Fish Biol. 2020 Feb;96(2):408-417. doi: 10.1111/jfb.14213. Epub 2019 Dec 17. J Fish Biol. 2020. PMID: 31755101 Free PMC article.
-
The northern shrimp (Pandalus borealis) offshore fishery in the Northeast Atlantic.Adv Mar Biol. 2007;52:147-266. doi: 10.1016/S0065-2881(06)52002-4. Adv Mar Biol. 2007. PMID: 17298891 Review.
-
A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation.Biol Rev Camb Philos Soc. 2007 May;82(2):173-211. doi: 10.1111/j.1469-185X.2006.00004.x. Biol Rev Camb Philos Soc. 2007. PMID: 17437557 Review.
Cited by
-
Advances in salmonid genetics-Insights from Coastwide and beyond.Evol Appl. 2024 Jun 17;17(6):e13732. doi: 10.1111/eva.13732. eCollection 2024 Jun. Evol Appl. 2024. PMID: 38887374 Free PMC article.
References
-
- Anderson, L. E. , & Lee, S. T. (2013). Untangling the recreational value of wild and hatchery Salmon. Marine Resource Economics, 28(2), 175–197. 10.5950/0738-1360-28.2.175 - DOI
-
- Aykanat, T. , Rasmussen, M. , Ozerov, M. , Niemelä, E. , Paulin, L. , Vähä, J.‐P. , Hindar, K. , Wennevik, V. , Pedersen, T. , Svenning, M.‐A. , & Primmer, C. R. (2020). Life‐history genomic regions explain differences in Atlantic salmon marine diet specialization. The Journal of Animal Ecology, 89, 2677–2691. - PubMed
-
- Ayllon, F. , Kjærner‐Semb, E. , Furmanek, T. , Wennevik, V. , Solberg, M. F. , Dahle, G. , Taranger, G. L. , Glover, K. A. , Sällman Almén, M. , Rubin, C. J. , Edvardsen, R. B. , & Wargelius, A. (2015). The vgll3 locus controls age at maturity in wild and domesticated Atlantic Salmon (Salmo salar L.) males. PLoS Genetics, 11(11), 1–15. 10.1371/journal.pgen.1005628 - DOI - PMC - PubMed
-
- Barson, N. J. , Aykanat, T. , Hindar, K. , Baranski, M. , Bolstad, G. H. , Fiske, P. , Jacq, C. , Jensen, A. J. , Johnston, S. E. , Karlsson, S. , Kent, M. , Moen, T. , Niemelä, E. , Nome, T. , Næsje, T. F. , Orell, P. , Romakkaniemi, A. , Sægrov, H. , Urdal, K. , … Primmer, C. R. (2015). Sex‐dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature, 528(7582), 405–408. 10.1038/nature16062 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

