Lipopolysaccharide promotes cancer cell migration and invasion through METTL3/PI3K/AKT signaling in human cholangiocarcinoma
- PMID: 38681552
- PMCID: PMC11053196
- DOI: 10.1016/j.heliyon.2024.e29683
Lipopolysaccharide promotes cancer cell migration and invasion through METTL3/PI3K/AKT signaling in human cholangiocarcinoma
Abstract
Purpose: As a major structural component of the outer membrane of Gram-negative bacteria, lipopolysaccharide (LPS) has been detected in the blood circulation and tissues in patients with chronic diseases and cancers, which plays a critical role in the tumor formation and progression. However, the biological role of LPS in human intrahepatic cholangiocarcinoma remains unclear. The aims of this study were to investigate the role of LPS in the malignant progression of intrahepatic cholangiocarcinoma.
Methods: The cell migration and invasion capacities of cholangiocarcinoma cell lines were evaluated by Boyden chamber assays. Expression levels of the key molecules involved in the PI3K/AKT signaling and METTL3 were detected by qPCR and western blot. The molecular mechanism by which LPS promotes the malignant behaviors was investigated by using siRNAs, plasmids and small molecule inhibitors.
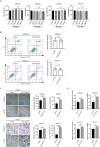
Results: In vitro experiments showed that exogenous LPS treatment promoted cell migration and invasion capacities in both QBC939 and HUCCT1 cell lines, while did not affect cell proliferation and apoptosis. Mechanistically, exogenous LPS treatment had been proved to induce the increased expression of METTL3 and activate the downstream PI3K/AKTsignaling pathway. In addition, suppression of METTL3 expression reduced cell proliferation, migration and invasion capacities in both cell lines. Furthermore, inhibition of METTL3 expression or inhibition of PI3K/AKT signaling decreased LPS-induced cell migration and invasion capacities. Moreover, knockdown of METTL3 or inhibition of METTL3 significantly inhibited LPS-induced activation of the PI3K/AKT signaling.
Conclusion: In general, these results suggest that the LPS-METTL3-PI3K/AKT signal axis promotes cell migration and invasion in ICC, which contributes to a reduced overall survival in patients with ICC. It may broaden the horizon of cancer therapy with potential therapeutic targets.
Keywords: Cell invasion; Intrahepatic cholangiocarcinoma; Lipopolysaccharide; METTL3; PI3K/AKT signaling.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Wang E.L., Qian Z.R., Nakasono M., Tanahashi T., Yoshimoto K., Bando Y., Kudo E., Shimada M., Sano T. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br. J. Cancer. 2010;102:908–915. doi: 10.1038/sj.bjc.6605558. - DOI - PMC - PubMed
-
- Killeen S.D., Wang J.H., Andrews E.J., Redmond H.P. Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator system. Br. J. Cancer. 2009;100:1589–1602. doi: 10.1038/sj.bjc.6604942. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

