Taurine attenuates activation of hepatic stellate cells by inhibiting autophagy and inducing ferroptosis
- PMID: 38681990
- PMCID: PMC11045481
- DOI: 10.3748/wjg.v30.i15.2143
Taurine attenuates activation of hepatic stellate cells by inhibiting autophagy and inducing ferroptosis
Abstract
Background: Liver fibrosis is a compensatory response during the tissue repair process in chronic liver injury, and finally leads to liver cirrhosis or even hepatocellular carcinoma. The pathogenesis of hepatic fibrosis is associated with the progressive accumulation of activated hepatic stellate cells (HSCs), which can transdifferentiate into myofibroblasts to produce an excess of the extracellular matrix (ECM). Myofibroblasts are the main source of the excessive ECM responsible for hepatic fibrosis. Therefore, activated hepatic stellate cells (aHSCs), the principal ECM producing cells in the injured liver, are a promising therapeutic target for the treatment of hepatic fibrosis.
Aim: To explore the effect of taurine on aHSC proliferation and the mechanisms involved.
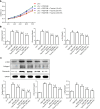
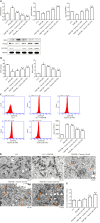
Methods: Human HSCs (LX-2) were randomly divided into five groups: Normal control group, platelet-derived growth factor-BB (PDGF-BB) (20 ng/mL) treated group, and low, medium, and high dosage of taurine (10 mmol/L, 50 mmol/L, and 100 mmol/L, respectively) with PDGF-BB (20 ng/mL) treated group. Cell Counting Kit-8 method was performed to evaluate the effect of taurine on the viability of aHSCs. Enzyme-linked immunosorbent assay was used to estimate the effect of taurine on the levels of reactive oxygen species (ROS), malondialdehyde, glutathione, and iron concentration. Transmission electron microscopy was applied to observe the effect of taurine on the autophagosomes and ferroptosis features in aHSCs. Quantitative real-time polymerase chain reaction and Western blot analysis were performed to detect the effect of taurine on the expression of α-SMA, Collagen I, Fibronectin 1, LC3B, ATG5, Beclin 1, PTGS2, SLC7A11, and p62.
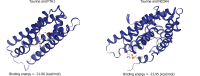
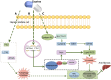
Results: Taurine promoted the death of aHSCs and reduced the deposition of the ECM. Treatment with taurine could alleviate autophagy in HSCs to inhibit their activation, by decreasing autophagosome formation, downregulating LC3B and Beclin 1 protein expression, and upregulating p62 protein expression. Meanwhile, treatment with taurine triggered ferroptosis and ferritinophagy to eliminate aHSCs characterized by iron overload, lipid ROS accumulation, glutathione depletion, and lipid peroxidation. Furthermore, bioinformatics analysis demonstrated that taurine had a direct targeting effect on nuclear receptor coactivator 4, exhibiting the best average binding affinity of -20.99 kcal/mol.
Conclusion: Taurine exerts therapeutic effects on liver fibrosis via mechanisms that involve inhibition of autophagy and trigger of ferroptosis and ferritinophagy in HSCs to eliminate aHSCs.
Keywords: Autophagy; Ferroptosis; Hepatic stellate cells; Molecular docking; Taurine.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

References
-
- Zheng Y, Zhao T, Wang J, Jiang R, Huang J, Li W. Curcumol alleviates liver fibrosis through inducing autophagy and ferroptosis in hepatic stellate cells. FASEB J. 2022;36:e22665. - PubMed
-
- Shao C, Xu H, Sun X, Huang Y, Guo W, He Y, Ye L, Wang Z, Huang J, Liang X, Zhang J. New Perspectives on Chinese Medicine in Treating Hepatic Fibrosis: Lipid Droplets in Hepatic Stellate Cells. Am J Chin Med. 2023;51:1413–1429. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

