Dexmedetomidine alleviates blood-brain barrier disruption in rats after cerebral ischemia-reperfusion by suppressing JNK and p38 MAPK signaling
- PMID: 38682172
- PMCID: PMC11058545
- DOI: 10.4196/kjpp.2024.28.3.239
Dexmedetomidine alleviates blood-brain barrier disruption in rats after cerebral ischemia-reperfusion by suppressing JNK and p38 MAPK signaling
Abstract
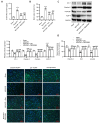
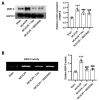
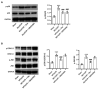
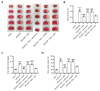
Dexmedetomidine displays multiple mechanisms of neuroprotection in ameliorating ischemic brain injury. In this study, we explored the beneficial effects of dexmedetomidine on blood-brain barrier (BBB) integrity and neuroinflammation in cerebral ischemia/reperfusion injury. Sprague-Dawley rats were subjected to middle cerebral artery occlusion (MCAO) for 1.5 h and reperfusion for 24 h to establish a rat model of cerebral ischemia/reperfusion injury. Dexmedetomidine (9 g/kg) was administered to rats 30 min after MCAO through intravenous injection, and SB203580 (a p38 MAPK inhibitor, 200 g/kg) was injected intraperitoneally 30 min before MCAO. Brain damages were evaluated by 2,3,5-triphenyltetrazolium chloride staining, hematoxylin-eosin staining, Nissl staining, and brain water content assessment. BBB permeability was examined by Evans blue staining. Expression levels of claudin-5, zonula occludens-1, occludin, and matrix metalloproteinase-9 (MMP-9) as well as M1/M2 phenotypes-associated markers were assessed using immunofluorescence, RT-qPCR, Western blotting, and gelatin zymography. Enzyme-linked immunosorbent assay was used to examine inflammatory cytokine levels. We found that dexmedetomidine or SB203580 attenuated infarct volume, brain edema, BBB permeability, and neuroinflammation, and promoted M2 microglial polarization after cerebral ischemia/reperfusion injury. Increased MMP-9 activity by ischemia/reperfusion injury was inhibited by dexmedetomidine or SB203580. Dexmedetomidine inhibited the activation of the ERK, JNK, and p38 MAPK pathways. Moreover, activation of JNK or p38 MAPK reversed the protective effects of dexmedetomidine against ischemic brain injury. Overall, dexmedetomidine ameliorated brain injury by alleviating BBB permeability and promoting M2 polarization in experimental cerebral ischemia/reperfusion injury model by inhibiting the activation of JNK and p38 MAPK pathways.
Keywords: Blood-brain barrier; Dexmedetomidine; Matrix metalloproteinase 9; Microglia; Middle cerebral artery occlusion; p38 mitogen-activated protein kinases.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Regulation of Blood-Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer's Disease and Brain Tumors.Molecules. 2025 May 28;30(11):2353. doi: 10.3390/molecules30112353. Molecules. 2025. PMID: 40509244 Free PMC article. Review.
-
The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion.Brain Res. 2014 Jan 28;1544:45-53. doi: 10.1016/j.brainres.2013.11.031. Epub 2013 Dec 6. Brain Res. 2014. PMID: 24316243
-
PD-1 mediates microglia polarization via the MAPK signaling pathway to protect blood-brain barrier function during cerebral ischemia/reperfusion.Brain Res Bull. 2024 Oct 1;216:111055. doi: 10.1016/j.brainresbull.2024.111055. Epub 2024 Aug 20. Brain Res Bull. 2024. PMID: 39173779
-
microRNA-21 Confers Neuroprotection Against Cerebral Ischemia-Reperfusion Injury and Alleviates Blood-Brain Barrier Disruption in Rats via the MAPK Signaling Pathway.J Mol Neurosci. 2018 May;65(1):43-53. doi: 10.1007/s12031-018-1067-5. Epub 2018 Apr 26. J Mol Neurosci. 2018. PMID: 29696468
-
All-Trans Retinoic Acid Ameliorates the Early Experimental Cerebral Ischemia-Reperfusion Injury in Rats by Inhibiting the Loss of the Blood-Brain Barrier via the JNK/P38MAPK Signaling Pathway.Neurochem Res. 2018 Jun;43(6):1283-1296. doi: 10.1007/s11064-018-2545-4. Epub 2018 May 25. Neurochem Res. 2018. PMID: 29802528
Cited by
-
Regulation of Blood-Brain Barrier Permeability via JNK Signaling Pathway: Mechanisms and Potential Therapeutic Strategies for Ischemic Stroke, Alzheimer's Disease and Brain Tumors.Molecules. 2025 May 28;30(11):2353. doi: 10.3390/molecules30112353. Molecules. 2025. PMID: 40509244 Free PMC article. Review.
-
Shikonin attenuates blood-brain barrier injury and oxidative stress in rats with subarachnoid hemorrhage by activating Sirt1/Nrf2/HO-1 signaling.Korean J Physiol Pharmacol. 2025 May 1;29(3):283-291. doi: 10.4196/kjpp.24.182. Korean J Physiol Pharmacol. 2025. PMID: 40254555 Free PMC article.
-
Molecular and biological approach to ischemia/reperfusion in diabetes conditions.Mol Biol Rep. 2025 Jun 21;52(1):620. doi: 10.1007/s11033-025-10686-x. Mol Biol Rep. 2025. PMID: 40544201 Review. No abstract available.
-
Amorfrutin A ameliorates cerebral ischemia/reperfsion injury in vivo and in vitro via modulating Nrf2/HO-1 signaling pathway.Korean J Physiol Pharmacol. 2025 Sep 1;29(5):547-557. doi: 10.4196/kjpp.24.304. Epub 2025 May 15. Korean J Physiol Pharmacol. 2025. PMID: 40368848 Free PMC article.
References
-
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. - DOI - PubMed
-
- Chapman SN, Mehndiratta P, Johansen MC, McMurry TL, Johnston KC, Southerland AM. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag. 2014;10:75–87. doi: 10.2147/VHRM.S39213. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

