Acute effects of a ketone monoester, whey protein, or their coingestion on mTOR trafficking and protein-protein colocalization in human skeletal muscle
- PMID: 38682238
- PMCID: PMC11371313
- DOI: 10.1152/ajpcell.00207.2024
Acute effects of a ketone monoester, whey protein, or their coingestion on mTOR trafficking and protein-protein colocalization in human skeletal muscle
Abstract
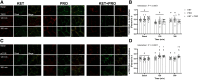
We recently demonstrated that acute oral ketone monoester intake induces a stimulation of postprandial myofibrillar protein synthesis rates comparable to that elicited following the ingestion of 10 g whey protein or their coingestion. The present investigation aimed to determine the acute effects of ingesting a ketone monoester, whey protein, or their coingestion on mechanistic target of rapamycin (mTOR)-related protein-protein colocalization and intracellular trafficking in human skeletal muscle. In a randomized, double-blind, parallel group design, 36 healthy recreationally active young males (age: 24.2 ± 4.1 yr) ingested either: 1) 0.36 g·kg-1 bodyweight of the ketone monoester (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (KET), 2) 10 g whey protein (PRO), or 3) the combination of both (KET + PRO). Muscle biopsies were obtained in the overnight postabsorptive state (basal conditions), and at 120 and 300 min in the postprandial period for immunofluorescence assessment of protein translocation and colocalization of mTOR-related signaling molecules. All treatments resulted in a significant (Interaction: P < 0.0001) decrease in tuberous sclerosis complex 2 (TSC2)-Ras homolog enriched in brain (Rheb) colocalization at 120 min versus basal; however, the decrease was sustained at 300 min versus basal (P < 0.0001) only in KET + PRO. PRO and KET + PRO increased (Interaction: P < 0.0001) mTOR-Rheb colocalization at 120 min versus basal; however, KET + PRO resulted in a sustained increase in mTOR-Rheb colocalization at 300 min that was greater than KET and PRO. Treatment intake increased mTOR-wheat germ agglutinin (WGA) colocalization at 120 and 300 min (Time: P = 0.0031), suggesting translocation toward the fiber periphery. These findings demonstrate that ketone monoester intake can influence the spatial mechanisms involved in the regulation of mTORC1 in human skeletal muscle.NEW & NOTEWORTHY We explored the effects of a ketone monoester (KET), whey protein (PRO), or their coingestion (KET + PRO) on mTOR-related protein-protein colocalization and intracellular trafficking in human muscle. All treatments decreased TSC2-Rheb colocalization at 120 minutes; however, KET + PRO sustained the decrease at 300 min. Only PRO and KET + PRO increased mTOR-Rheb colocalization; however, the increase at 300 min was greater in KET + PRO. Treatment intake increased mTOR-WGA colocalization, suggesting translocation to the fiber periphery. Ketone bodies influence the spatial regulation of mTOR.
Keywords: Rheb; TSC2; immunofluorescence; ketone bodies; β-hydroxybutyrate.
Conflict of interest statement
S.A.S is an employee of Iovate Health Sciences, a manufacturer of sports nutrition products, but did not provide nor develop the investigational supplements. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures

References
-
- Hannaian SJ, Hodson N, Abou Sawan S, Mazzulla M, Kato H, Matsunaga K, Waskiw-Ford M, Duncan J, Kumbhare DA, Moore DR. Leucine-enriched amino acids maintain peripheral mTOR-Rheb localization independent of myofibrillar protein synthesis and mTORC1 signaling postexercise. J Appl Physiol (1985) 129: 133–143, 2020. doi:10.1152/japplphysiol.00241.2020. - DOI - PMC - PubMed
-
- Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Rüegg MA, Hamilton DL, Phillips SM, Philp A. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 313: C604–C611, 2017. doi:10.1152/ajpcell.00176.2017. - DOI - PMC - PubMed
-
- Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, Philp A, Li Z, Paluska SA, Burd NA, Moore DR. Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep 6: e13628, 2018. doi:10.14814/phy2.13628. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

