Optical coherence tomography-guided Brillouin microscopy highlights regional tissue stiffness differences during anterior neural tube closure in the Mthfd1l murine mutant
- PMID: 38682273
- PMCID: PMC11165724
- DOI: 10.1242/dev.202475
Optical coherence tomography-guided Brillouin microscopy highlights regional tissue stiffness differences during anterior neural tube closure in the Mthfd1l murine mutant
Abstract
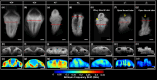
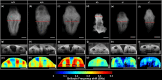
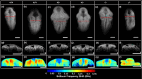
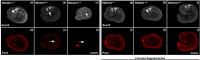
Neurulation is a highly synchronized biomechanical process leading to the formation of the brain and spinal cord, and its failure leads to neural tube defects (NTDs). Although we are rapidly learning the genetic mechanisms underlying NTDs, the biomechanical aspects are largely unknown. To understand the correlation between NTDs and tissue stiffness during neural tube closure (NTC), we imaged an NTD murine model using optical coherence tomography (OCT), Brillouin microscopy and confocal fluorescence microscopy. Here, we associate structural information from OCT with local stiffness from the Brillouin signal of embryos undergoing neurulation. The stiffness of neuroepithelial tissues in Mthfd1l null embryos was significantly lower than that of wild-type embryos. Additionally, exogenous formate supplementation improved tissue stiffness and gross embryonic morphology in nullizygous and heterozygous embryos. Our results demonstrate the significance of proper tissue stiffness in normal NTC and pave the way for future studies on the mechanobiology of normal and abnormal embryonic development.
Keywords: Biomechanics; Brillouin microscopy; Mouse; Neural tube closure; Neural tube defects.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests M.S. and K.V.L. have a financial interest in ElastEye, which is unrelated to this work. All other authors declare they have no competing interests.
Figures



Similar articles
-
Deletion of neural tube defect-associated gene Mthfd1l causes reduced cranial mesenchyme density.Birth Defects Res. 2019 Nov 15;111(19):1520-1534. doi: 10.1002/bdr2.1591. Epub 2019 Sep 13. Birth Defects Res. 2019. PMID: 31518072 Free PMC article.
-
Deletion of the neural tube defect-associated gene Mthfd1l disrupts one-carbon and central energy metabolism in mouse embryos.J Biol Chem. 2018 Apr 20;293(16):5821-5833. doi: 10.1074/jbc.RA118.002180. Epub 2018 Feb 26. J Biol Chem. 2018. PMID: 29483189 Free PMC article.
-
Deletion of Mthfd1l causes embryonic lethality and neural tube and craniofacial defects in mice.Proc Natl Acad Sci U S A. 2013 Jan 8;110(2):549-54. doi: 10.1073/pnas.1211199110. Epub 2012 Dec 24. Proc Natl Acad Sci U S A. 2013. PMID: 23267094 Free PMC article.
-
Mitochondrial one-carbon metabolism and neural tube defects.Birth Defects Res A Clin Mol Teratol. 2014 Aug;100(8):576-83. doi: 10.1002/bdra.23268. Epub 2014 Jul 1. Birth Defects Res A Clin Mol Teratol. 2014. PMID: 24985542 Review.
-
Genetic backgrounds and modifier genes of NTD mouse models: An opportunity for greater understanding of the multifactorial etiology of neural tube defects.Birth Defects Res. 2017 Jan 30;109(2):140-152. doi: 10.1002/bdra.23554. Birth Defects Res. 2017. PMID: 27768235 Review.
References
-
- Ambekar, Y. S., Singh, M., Zhang, J., Nair, A., Aglyamov, S. R., Scarcelli, G. and Larin, K. V. (2020). Multimodal quantitative optical elastography of the crystalline lens with optical coherence elastography and Brillouin microscopy. Biomed. Opt. Express 11, 2041-2051. 10.1364/BOE.387361 - DOI - PMC - PubMed
-
- Ambekar, Y. S., Singh, M., Schill, A. W., Zhang, J., Zevallos-Delgado, C., Khajavi, B., Aglyamov, S. R., Finnell, R. H., Scarcelli, G. and Larin, K. V. (2022). Multimodal imaging system combining optical coherence tomography and Brillouin microscopy for neural tube imaging. Opt. Lett. 47, 1347-1350. 10.1364/OL.453996 - DOI - PMC - PubMed
-
- Armistead, J., Patel, N., Wu, X., Hemming, R., Chowdhury, B., Basra, G. S., Del Bigio, M. R., Ding, H. and Triggs-Raine, B. (2015). Growth arrest in the ribosomopathy, Bowen-Conradi syndrome, is due to dramatically reduced cell proliferation and a defect in mitotic progression. Biochim. Biophys. Acta 1852, 1029-1037. 10.1016/j.bbadis.2015.02.007 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

