ABCG2-Expressing Clonal Repopulating Endothelial Cells Serve to Form and Maintain Blood Vessels
- PMID: 38682338
- PMCID: PMC11300167
- DOI: 10.1161/CIRCULATIONAHA.122.061833
ABCG2-Expressing Clonal Repopulating Endothelial Cells Serve to Form and Maintain Blood Vessels
Abstract
Background: Most organs are maintained lifelong by resident stem/progenitor cells. During development and regeneration, lineage-specific stem/progenitor cells can contribute to the growth or maintenance of different organs, whereas fully differentiated mature cells have less regenerative potential. However, it is unclear whether vascular endothelial cells (ECs) are also replenished by stem/progenitor cells with EC-repopulating potential residing in blood vessels. It has been reported recently that some EC populations possess higher clonal proliferative potential and vessel-forming capacity compared with mature ECs. Nevertheless, a marker to identify vascular clonal repopulating ECs (CRECs) in murine and human individuals is lacking, and, hence, the mechanism for the proliferative, self-renewal, and vessel-forming potential of CRECs is elusive.
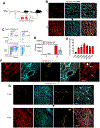
Methods: We analyzed colony-forming, self-renewal, and vessel-forming potential of ABCG2 (ATP binding cassette subfamily G member 2)-expressing ECs in human umbilical vessels. To study the contribution of Abcg2-expressing ECs to vessel development and regeneration, we developed Abcg2CreErt2;ROSA TdTomato mice and performed lineage tracing during mouse development and during tissue regeneration after myocardial infarction injury. RNA sequencing and chromatin methylation chromatin immunoprecipitation followed by sequencing were conducted to study the gene regulation in Abcg2-expressing ECs.
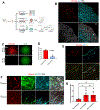
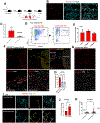
Results: In human and mouse vessels, ECs with higher ABCG2 expression (ABCECs) possess higher clonal proliferative potential and in vivo vessel-forming potential compared with mature ECs. These cells could clonally contribute to vessel formation in primary and secondary recipients after transplantation. These features of ABCECs meet the criteria of CRECs. Results from lineage tracing experiments confirm that Abcg2-expressing CRECs (AbcCRECs) contribute to arteries, veins, and capillaries in cardiac tissue development and vascular tissue regeneration after myocardial infarction. Transcriptome and epigenetic analyses reveal that a gene expression signature involved in angiogenesis and vessel development is enriched in AbcCRECs. In addition, various angiogenic genes, such as Notch2 and Hey2, are bivalently modified by trimethylation at the 4th and 27th lysine residue of histone H3 (H3K4me3 and H3K27me3) in AbcCRECs.
Conclusions: These results are the first to establish that a single prospective marker identifies CRECs in mice and human individuals, which holds promise to provide new cell therapies for repair of damaged vessels in patients with endothelial dysfunction.
Keywords: angiogenesis; blood vessels; developmental biology; endothelial cells.
Conflict of interest statement
Dr Rafii is a cofounder of and a nonpaid consultant to Angiocrine Bioscience. Dr Yoder is a scientific cofounder of Vascugen.
Figures


References
-
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature reviews Molecular cell biology. 2014;15:19–33. - PubMed
-
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D and Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. - PubMed
-
- Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A and Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. - PubMed
-
- Naito H, Wakabayashi T, Kidoya H, Muramatsu F, Takara K, Eino D, Yamane K, Iba T and Takakura N. Endothelial Side Population Cells Contribute to Tumor Angiogenesis and Antiangiogenic Drug Resistance. Cancer research. 2016;76:3200–10. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK136327/DK/NIDDK NIH HHS/United States
- R01 EY012601/EY/NEI NIH HHS/United States
- R21 AI178153/AI/NIAID NIH HHS/United States
- S10 OD012270/OD/NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U54 DK106846/DK/NIDDK NIH HHS/United States
- P30 EY003039/EY/NEI NIH HHS/United States
- RC2 DK114777/DK/NIDDK NIH HHS/United States
- R35 HL150809/HL/NHLBI NIH HHS/United States
- P30 CA082709/CA/NCI NIH HHS/United States
- R01 HL171277/HL/NHLBI NIH HHS/United States
- U01 AI138329/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

