Functional redundancy in tRNA dihydrouridylation
- PMID: 38682613
- PMCID: PMC11162810
- DOI: 10.1093/nar/gkae325
Functional redundancy in tRNA dihydrouridylation
Erratum in
-
Correction to 'Functional redundancy in tRNA dihydrouridylation'.Nucleic Acids Res. 2025 Feb 27;53(5):gkaf185. doi: 10.1093/nar/gkaf185. Nucleic Acids Res. 2025. PMID: 40052828 Free PMC article. No abstract available.
Abstract
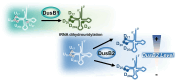
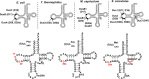
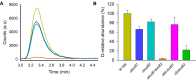
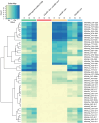
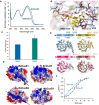
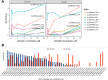
Dihydrouridine (D) is a common modified base found predominantly in transfer RNA (tRNA). Despite its prevalence, the mechanisms underlying dihydrouridine biosynthesis, particularly in prokaryotes, have remained elusive. Here, we conducted a comprehensive investigation into D biosynthesis in Bacillus subtilis through a combination of genetic, biochemical, and epitranscriptomic approaches. Our findings reveal that B. subtilis relies on two FMN-dependent Dus-like flavoprotein homologs, namely DusB1 and DusB2, to introduce all D residues into its tRNAs. Notably, DusB1 exhibits multisite enzyme activity, enabling D formation at positions 17, 20, 20a and 47, while DusB2 specifically catalyzes D biosynthesis at positions 20 and 20a, showcasing a functional redundancy among modification enzymes. Extensive tRNA-wide D-mapping demonstrates that this functional redundancy impacts the majority of tRNAs, with DusB2 displaying a higher dihydrouridylation efficiency compared to DusB1. Interestingly, we found that BsDusB2 can function like a BsDusB1 when overexpressed in vivo and under increasing enzyme concentration in vitro. Furthermore, we establish the importance of the D modification for B. subtilis growth at suboptimal temperatures. Our study expands the understanding of D modifications in prokaryotes, highlighting the significance of functional redundancy in this process and its impact on bacterial growth and adaptation.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures