Epstein-Barr virus-associated post-transplant lymphoproliferative disorders in pediatric transplantation: A prospective multicenter study in the United States
- PMID: 38682750
- PMCID: PMC11115376
- DOI: 10.1111/petr.14763
Epstein-Barr virus-associated post-transplant lymphoproliferative disorders in pediatric transplantation: A prospective multicenter study in the United States
Abstract
Background: Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disorders (PTLD) is the most common malignancy in children after transplant; however, difficulties for early detection may worsen the prognosis.
Methods: The prospective, multicenter, study enrolled 944 children (≤21 years of age). Of these, 872 received liver, heart, kidney, intestinal, or multivisceral transplants in seven US centers between 2014 and 2019 (NCT02182986). In total, 34 pediatric EBV+ PTLD (3.9%) were identified by biopsy. Variables included sex, age, race, ethnicity, transplanted organ, EBV viral load, pre-transplant EBV serology, immunosuppression, response to chemotherapy and rituximab, and histopathological diagnosis.
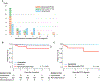
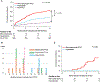
Results: The uni-/multivariable competing risk analyses revealed the combination of EBV-seropositive donor and EBV-naïve recipient (D+R-) was a significant risk factor for PTLD development (sub-hazard ratio: 2.79 [1.34-5.78], p = .006) and EBV DNAemia (2.65 [1.72-4.09], p < .001). Patients with D+R- were significantly more associated with monomorphic/polymorphic PTLD than those with the other combinations (p = .02). Patients with monomorphic/polymorphic PTLD (n = 21) had significantly more EBV DNAemia than non-PTLD patients (p < .001) and an earlier clinical presentation of PTLD than patients with hyperplasias (p < .001), within 6-month post-transplant. Among non-liver transplant recipients, monomorphic/polymorphic PTLD were significantly more frequent than hyperplasias in patients ≥5 years of age at transplant (p = .01).
Conclusions: D+R- is a risk factor for PTLD and EBV DNAemia and associated with the incidence of monomorphic/polymorphic PTLD. Intensive follow-up of EBV viral load within 6-month post-transplant, especially for patients with D+R- and/or non-liver transplant recipients ≥5 years of age at transplant, may help detect monomorphic/polymorphic PTLD early in pediatric transplant.
Keywords: Epstein–Barr virus; PTLD; pediatric transplantation.
© 2024 The Authors. Pediatric Transplantation published by Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest Statement:
Author Brian Armstrong was employed by Rho. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. - PubMed
-
- Dierickx D, Habermann TM. Post-Transplantation Lymphoproliferative Disorders in Adults. N Engl J Med. 2018;378(6):549–562. - PubMed
-
- Dharnidharka VR. Comprehensive review of post-organ transplant hematologic cancers. Am J Transplant. 2018;18(3):537–549. - PubMed
-
- Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(2):222–230. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

