Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications
- PMID: 38683014
- PMCID: PMC11237673
- DOI: 10.1128/spectrum.00073-24
Comparative evaluation of RT-PCR and antigen-based rapid diagnostic tests (Ag-RDTs) for SARS-CoV-2 detection: performance, variant specificity, and clinical implications
Abstract
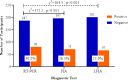
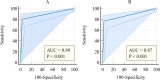
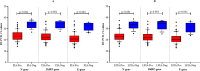
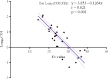
The COVID-19 pandemic has highlighted the critical need for accurate and efficient diagnostic tools for detecting severe acute respiratory coronavirus 2 (SARS-CoV-2) infections. This study presents a comparison of two diagnostic tests: RT-PCR and antigen detection rapid diagnostic tests (Ag-RDTs). This study focused on their performance, variant specificity, and their clinical implications. A simultaneous testing of 268 samples was carried out for SARS-CoV-2 using RT-PCR and Ag-RDTs [flourescence immunoassay (FIA) and lateral flow immunoassay (LFIA)]. Viral load was quantified, and variant identification was performed using a PCR-based assay. The prevalence was found to be 30.2% using reverse transcription PCR (RT-PCR), 26.5% using FIA, and 25% using LFIA. When comparing the FIA and LFIA, the overall diagnostic performance was found to be 80.25% vs 76.54%, 96.79% vs 97.33%, 91.55% vs 90.51%, and 91.88% vs 92.56% for sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), respectively. Both Ag-RDTs showed a strong agreement with RT-PCR (κ = 0.78-0.80). The overall accuracies of the FIA and LFIA were 92.41% and 92.13%, respectively. The FIA showed higher sensitivity (73.68%) and PPV (92.08%) than the LFIA (65.79% and 90.56%, respectively) in asymptomatic patients. At low Ct values (<25), both Ag-RDTs had 100% sensitivity, but the sensitivity reduced to 31.82% for FIA and 27.27% for LFIA at Ct values > 30. The diagnostic sensitivity of FIA compared to LFIA for detecting the Alpha variant was 78.85% vs. 69.23% and 72.22% vs. 83.33% for the Delta variant. Both Ag-RDTs had 100% sensitivity for detecting Omicron. Both Ag-RDTs performed well in patients with high viral loads and Omicron variant infections compared to those infected with Alpha and Delta variants. This study confirms the comparable performance of RT-PCR and Ag-RDTs, specifically FIA and LFIA, for SARS-CoV-2 detection. The FIA showed higher sensitivity and PPV in asymptomatic cases, while both Ag-RDTs exhibited strong agreement with RT-PCR results. Notably, Ag-RDTs, particularly FIA, proved effective in detecting the Omicron variant and cases with high viral loads, highlighting their potential clinical utility in managing the COVID-19 pandemic.IMPORTANCEThis study is of utmost importance in providing effective responses to manage the COVID-19 pandemic. It rigorously compares the diagnostic accuracy, variant specificity, and practical considerations of reverse transcription PCR (RT-PCR) and antigen detection rapid diagnostic tests (Ag-RDTs) for severe acute respiratory coronavirus 2 (SARS-CoV-2), answering critical questions. The results of this study will help healthcare professionals choose the appropriate testing methods, allocate resources effectively, and enhance public health strategies. Given the evolution of the virus, understanding the performance of these diagnostic tools is crucial to adapting to emerging variants. Additionally, the study provides insights into logistical challenges and accessibility issues, which will contribute to refining testing workflows, particularly in resource-limited settings. Ultimately, the study's impact extends to global healthcare, providing valuable information for policymakers, clinicians, and public health officials as they work together for mitigating the impact of the pandemic.
Keywords: COVID-19; RDT; RNA; RT-PCR; SARS-CoV-2; antigen; viral load.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Taqman PACMAN: a simple molecular approach for positive rapid antigen test confirmation during periods of low prevalence.Microbiol Spectr. 2024 May 2;12(5):e0407323. doi: 10.1128/spectrum.04073-23. Epub 2024 Apr 3. Microbiol Spectr. 2024. PMID: 38567975 Free PMC article.
-
Performance of rapid antigen tests to detect SARS-CoV-2 variant diversity and correlation with viral culture positivity: implication for diagnostic development and future public health strategies.mBio. 2024 Dec 11;15(12):e0273724. doi: 10.1128/mbio.02737-24. Epub 2024 Oct 31. mBio. 2024. PMID: 39480114 Free PMC article.
-
Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads.Viruses. 2021 Oct 14;13(10):2071. doi: 10.3390/v13102071. Viruses. 2021. PMID: 34696501 Free PMC article.
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis.PLoS Med. 2021 Aug 12;18(8):e1003735. doi: 10.1371/journal.pmed.1003735. eCollection 2021 Aug. PLoS Med. 2021. PMID: 34383750 Free PMC article.
References
-
- Quashie PK, Mutungi JK, Dzabeng F, Oduro-Mensah D, Opurum PC, Tapela K, Udoakang AJ, Asante I, Paemka L, Kumi-Ansah F, Quaye O, Amoako E, Armah R, Kilba C, Boateng NA, Ofori M, Kyei GB, Bediako Y, Ndam N, Abugri J, Ansah P, Ampofo WK, Mutapi F, Awandare GA, WACCBIP COVID-19 Team . 2021. Trends of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody prevalence in selected regions across Ghana. Wellcome Open Res 6:173. doi:10.12688/wellcomeopenres.16890.1 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

