Tyrosine kinase 2 modulates splenic B cells through type I IFN and TLR7 signaling
- PMID: 38683377
- PMCID: PMC11058799
- DOI: 10.1007/s00018-024-05234-y
Tyrosine kinase 2 modulates splenic B cells through type I IFN and TLR7 signaling
Erratum in
-
Correction: Tyrosine kinase 2 modulates splenic B cells through type I IFN and TLR7 signaling.Cell Mol Life Sci. 2024 Nov 15;81(1):455. doi: 10.1007/s00018-024-05482-y. Cell Mol Life Sci. 2024. PMID: 39545991 Free PMC article. No abstract available.
Abstract
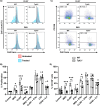
Tyrosine kinase 2 (TYK2) is involved in type I interferon (IFN-I) signaling through IFN receptor 1 (IFNAR1). This signaling pathway is crucial in the early antiviral response and remains incompletely understood on B cells. Therefore, to understand the role of TYK2 in B cells, we studied these cells under homeostatic conditions and following in vitro activation using Tyk2-deficient (Tyk2-/-) mice. Splenic B cell subpopulations were altered in Tyk2-/- compared to wild type (WT) mice. Marginal zone (MZ) cells were decreased and aged B cells (ABC) were increased, whereas follicular (FO) cells remained unchanged. Likewise, there was an imbalance in transitional B cells in juvenile Tyk2-/- mice. RNA sequencing analysis of adult MZ and FO cells isolated from Tyk2-/- and WT mice in homeostasis revealed altered expression of IFN-I and Toll-like receptor 7 (TLR7) signaling pathway genes. Flow cytometry assays corroborated a lower expression of TLR7 in MZ B cells from Tyk2-/- mice. Splenic B cell cultures showed reduced proliferation and differentiation responses after activation with TLR7 ligands in Tyk2-/- compared to WT mice, with a similar response to lipopolysaccharide (LPS) or anti-CD40 + IL-4. IgM, IgG, IL-10 and IL-6 secretion was also decreased in Tyk2-/- B cell cultures. This reduced response of the TLR7 pathway in Tyk2-/- mice was partially restored by IFNα addition. In conclusion, there is a crosstalk between TYK2 and TLR7 mediated by an IFN-I feedback loop, which contributes to the establishment of MZ B cells and to B cell proliferation and differentiation.
Keywords: Aged B cells; Follicular B cells; IFNα; Marginal zone B cells; TYK2-deficient mice; Toll-like receptor 7 (TLR7); Type I IFN; Tyrosine kinase 2 (TYK2); β receptor.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures





References
-
- Victora GD, Nussenzweig MC (2012) Germinal Centers. Annu Rev Immunol 30:429–457. 10.1146/annurev-immunol-020711-075032 - PubMed
-
- Martin F, Oliver AM, Kearney JF (2001) Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617–629. 10.1016/s1074-7613(01)00129-7 - PubMed
MeSH terms
Substances
Grants and funding
- PI19/00096/Ministerio de Ciencia e Innovación
- PI22/00428/Ministerio de Ciencia e Innovación
- RTI2018-099114-B-I00/Ministerio de Ciencia e Innovación
- RTI2018-099114-B-I00/Ministerio de Ciencia e Innovación
- P2022/BMD-7274/Dirección General de Innovación e Investigación Tecnológica de la Comunidad de Madrid
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

