Does the introduction of an infliximab biosimilar always result in savings for hospitals? A descriptive study using real-world data
- PMID: 38683413
- PMCID: PMC11059762
- DOI: 10.1186/s13561-024-00507-5
Does the introduction of an infliximab biosimilar always result in savings for hospitals? A descriptive study using real-world data
Abstract
Background: Biosimilars are biologic drugs that have the potential to increase the efficiency of healthcare spending and curb drug-related cost increases. However, their introduction into hospital formularies through initiatives such as non-medical switching must be carefully orchestrated so as not to cause treatment discontinuation or result in increased health resource utilization, such as additional visits or laboratory tests, among others. This retrospective cohort study aims to assess the impact of the introduction of CT-P13 on the healthcare expenditures of patients who were treated with originator infliximab or CT-P13.
Methods: Gastroenterology, immunoallergology and rheumatology patients treated between September 2017 and December 2020 at a university hospital in Western Switzerland were included and divided into seven cohorts, based on their treatment pathway (i.e., use and discontinuation of CT-P13 and/or originator infliximab). Costs in Swiss francs were obtained from the hospital's cost accounting department and length of stay was extracted from inpatient records. Comparisons of costs and length of stay between cohorts were calculated by bootstrapping.
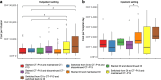
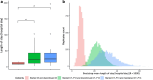
Results: Sixty immunoallergology, 84 rheumatology and 114 gastroenterology patients were included. Inpatient and outpatient costs averaged (sd) CHF 1,611 (1,020) per hospital day and CHF 4,991 (6,931) per infusion, respectively. The mean (sd) length of stay was 20 (28) days. Although immunoallergology and rheumatology patients had higher average costs than gastroenterology patients, differences in costs and length of stay were not formally explained by treatment pathway. Differences in health resource utilization were marginal.
Conclusions: The introduction of CT-P13 and the disruption of patient treatment management were not associated with differences in average outpatient and inpatient costs and length of stay, in contrast to the results reported in the rest of the literature. Future research should focus on the cost-effectiveness of non-medical switching policies and the potential benefits for patients.
Keywords: Biosimilars; CT-P13; Health resource utilization; Hospital formulary; Infliximab; Non-medical switching; Real-world data; Savings; TNF-α inhibitors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 non-inferiority trial.Lancet Gastroenterol Hepatol. 2018 Jun;3(6):404-412. doi: 10.1016/S2468-1253(18)30082-7. Epub 2018 Mar 30. Lancet Gastroenterol Hepatol. 2018. PMID: 29606564 Clinical Trial.
-
Switching from originator to biosimilar infliximab - real world data of a prospective 18 months follow-up of a single-centre IBD population.Scand J Gastroenterol. 2018 Jun;53(6):692-699. doi: 10.1080/00365521.2018.1463391. Epub 2018 May 31. Scand J Gastroenterol. 2018. PMID: 29852793
-
Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial.Lancet. 2017 Jun 10;389(10086):2304-2316. doi: 10.1016/S0140-6736(17)30068-5. Epub 2017 May 11. Lancet. 2017. PMID: 28502609 Clinical Trial.
-
Biosimilars in ulcerative colitis: When and for who?Best Pract Res Clin Gastroenterol. 2018 Feb-Apr;32-33:35-42. doi: 10.1016/j.bpg.2018.05.003. Epub 2018 May 26. Best Pract Res Clin Gastroenterol. 2018. PMID: 30060937 Review.
-
Discontinuation and Switchback After Non-Medical Switching from Originator Tumor Necrosis Factor Alpha (TNF) Inhibitors to Biosimilars: A Meta-Analysis of Real-World Studies from 2012 to 2018.Adv Ther. 2022 Aug;39(8):3711-3734. doi: 10.1007/s12325-022-02173-7. Epub 2022 Jun 23. Adv Ther. 2022. PMID: 35737227 Free PMC article.
References
-
- European Medicine Agency (EMA). Biological medicine 2022 [Available from: https://www.ema.europa.eu/en/glossary/biological-medicine.
-
- U.S. Food & Drug Administration (FDA). Biological product definitions 2020 [Available from: https://www.fda.gov/media/108557/download.
-
- Yasmeen N, Sawyer LM, Malottki K, Levin L, Didriksen Apol E, Jemec GB. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2022;33(1):204–18. doi: 10.1080/09546634.2020.1743811. - DOI - PubMed
-
- Murage MJ, Tongbram V, Feldman SR, Malatestinic WN, Larmore CJ, Muram TM, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence. 2018;12:1483–503. doi: 10.2147/PPA.S167508. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

