PB006: A Natalizumab Biosimilar
- PMID: 38683493
- PMCID: PMC11371843
- DOI: 10.1007/s40261-024-01360-4
PB006: A Natalizumab Biosimilar
Erratum in
-
Correction to: PB006: A Natalizumab Biosimilar.Clin Drug Investig. 2024 Sep;44(9):729. doi: 10.1007/s40261-024-01388-6. Clin Drug Investig. 2024. PMID: 39225908 Free PMC article. No abstract available.
Abstract
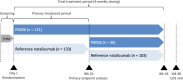
PB006 (Tyruko®) is the first biosimilar of the reference monoclonal anti-α4-integrin antibody natalizumab. It is approved for use in the same indications for which reference natalizumab is approved, as a single disease-modifying therapy in adults with highly active relapsing-remitting multiple sclerosis (RRMS). PB006 has similar physicochemical and pharmacodynamic properties to those of reference natalizumab, and the pharmacokinetic similarity of the agents has been demonstrated in a study in healthy subjects. PB006 demonstrated clinical efficacy similar to that of reference natalizumab in patients with RRMS, and was generally well tolerated in this population. The tolerability, safety and immunogenicity profiles of PB006 were similar to those of reference natalizumab, and switching from reference natalizumab to PB006 appeared to have no impact on tolerability or immunogenicity. The role of reference natalizumab in the management of RRMS is well established and PB006 provides an effective biosimilar alternative for patients requiring natalizumab therapy.
© 2024. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
During the peer review process, the manufacturer of PB006 was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit. Matt Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Figures
Similar articles
-
Pharmacokinetic and pharmacodynamic similarity of biosimilar natalizumab (PB006) to its reference medicine: a randomized controlled trial.Expert Opin Biol Ther. 2023 Jul-Dec;23(12):1287-1297. doi: 10.1080/14712598.2023.2290530. Epub 2023 Dec 28. Expert Opin Biol Ther. 2023. PMID: 38044885 Clinical Trial.
-
Efficacy and Safety of Proposed Biosimilar Natalizumab (PB006) in Patients With Relapsing-Remitting Multiple Sclerosis: The Antelope Phase 3 Randomized Clinical Trial.JAMA Neurol. 2023 Mar 1;80(3):298-307. doi: 10.1001/jamaneurol.2022.5007. JAMA Neurol. 2023. PMID: 36689214 Free PMC article. Clinical Trial.
-
Introducing the Biosimilar Paradigm to Neurology: The Totality of Evidence for the First Biosimilar Natalizumab.BioDrugs. 2024 Nov;38(6):755-767. doi: 10.1007/s40259-024-00671-4. Epub 2024 Sep 30. BioDrugs. 2024. PMID: 39343860 Free PMC article. Review.
-
Comparative immunogenicity assessment of biosimilar natalizumab to its reference medicine: a matching immunogenicity profile.Front Immunol. 2024 Dec 19;15:1414304. doi: 10.3389/fimmu.2024.1414304. eCollection 2024. Front Immunol. 2024. PMID: 39749348 Free PMC article. Clinical Trial.
-
AVT02: An Adalimumab Biosimilar.Clin Drug Investig. 2022 Oct;42(10):875-878. doi: 10.1007/s40261-022-01196-w. Epub 2022 Oct 1. Clin Drug Investig. 2022. PMID: 36181655 Free PMC article. Review.
Cited by
-
Cost-consequence analysis of early vs. delayed natalizumab use in highly active relapsing-remitting multiple sclerosis: a simulation study.J Neurol. 2025 Jan 17;272(2):153. doi: 10.1007/s00415-024-12723-4. J Neurol. 2025. PMID: 39821478 Free PMC article.
References
-
- European Medicines Agency. Tyruko: EU summary of product characteristics. 2023. https://www.ema.europa.eu. Accessed 02 Apr 2024.
-
- European Medicines Agency. Tyruko: European public assessment report. 2023. https://www.ema.europa.eu. Accessed 02 Apr 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources