Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer: The Alliance A011502 Randomized Trial
- PMID: 38683596
- PMCID: PMC11059055
- DOI: 10.1001/jama.2024.4840
Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer: The Alliance A011502 Randomized Trial
Abstract
Importance: Observational studies of survivors of breast cancer and prospective trials of aspirin for cardiovascular disease suggest improved breast cancer survival among aspirin users, but prospective studies of aspirin to prevent breast cancer recurrence are lacking.
Objective: To determine whether aspirin decreases the risk of invasive cancer events among survivors of breast cancer.
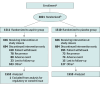
Design, setting, and participants: A011502, a phase 3, randomized, placebo-controlled, double-blind trial conducted in the United States and Canada with 3020 participants who had high-risk nonmetastatic breast cancer, enrolled participants from 534 sites from January 6, 2017, through December 4, 2020, with follow-up to March 4, 2023.
Interventions: Participants were randomized (stratified for hormone receptor status [positive vs negative], body mass index [≤30 vs >30], stage II vs III, and time since diagnosis [<18 vs ≥18 months]) to receive 300 mg of aspirin (n = 1510) or placebo once daily (n = 1510) for 5 years.
Main outcomes and measures: The primary outcome was invasive disease-free survival. Overall survival was a key secondary outcome.
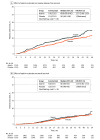
Results: A total of 3020 participants were randomized when the data and safety monitoring committee recommended suspending the study at the first interim analysis because the hazard ratio had crossed the prespecified futility bound. By median follow-up of 33.8 months (range, 0.1-72.6 months), 253 invasive disease-free survival events were observed (141 in the aspirin group and 112 in the placebo group), yielding a hazard ratio of 1.27 (95% CI, 0.99-1.63; P = .06). All invasive disease-free survival events, including death, invasive progression (both distant and locoregional), and new primary events, were numerically higher in the aspirin group, although the differences were not statistically significant. There was no difference in overall survival (hazard ratio, 1.19; 95% CI, 0.82-1.72). Rates of grades 3 and 4 adverse events were similar in both groups.
Conclusion and relevance: Among participants with high-risk nonmetastatic breast cancer, daily aspirin therapy did not improve risk of breast cancer recurrence or survival in early follow-up. Despite its promise and wide availability, aspirin should not be recommended as an adjuvant breast cancer treatment.
Trial registration: ClinicalTrials.gov Identifier: NCT02927249.
Conflict of interest statement
Figures

Comment in
-
The Aspirin Conundrum-Navigating Negative Results, Age, Aging Dynamics, and Equity.JAMA. 2024 May 28;331(20):1709-1711. doi: 10.1001/jama.2024.4828. JAMA. 2024. PMID: 38683570 Free PMC article. No abstract available.
-
Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer.JAMA. 2024 Oct 1;332(13):1112. doi: 10.1001/jama.2024.15494. JAMA. 2024. PMID: 39235794 No abstract available.
-
Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer.JAMA. 2024 Oct 1;332(13):1112-1113. doi: 10.1001/jama.2024.15488. JAMA. 2024. PMID: 39235804 No abstract available.
-
Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer.JAMA. 2024 Oct 1;332(13):1113. doi: 10.1001/jama.2024.15491. JAMA. 2024. PMID: 39235818 No abstract available.
-
Aspirin vs Placebo as Adjuvant Therapy for Breast Cancer.JAMA. 2024 Oct 1;332(13):1111-1112. doi: 10.1001/jama.2024.15497. JAMA. 2024. PMID: 39235821 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

