Hepatocellular Carcinoma Screening in a Contemporary Cohort of At-Risk Patients
- PMID: 38683607
- PMCID: PMC11059036
- DOI: 10.1001/jamanetworkopen.2024.8755
Hepatocellular Carcinoma Screening in a Contemporary Cohort of At-Risk Patients
Abstract
Importance: Cohort studies demonstrating an association of hepatocellular carcinoma (HCC) screening with reduced mortality are prone to lead-time and length-time biases.
Objective: To characterize the clinical benefits of HCC screening, adjusting for lead-time and length-time biases, in a diverse, contemporary cohort of at-risk patients.
Design, setting, and participants: This retrospective cohort study of patients with HCC was conducted between January 2008 and December 2022 at 2 large US health systems. Data analysis was performed from September to November 2023.
Main outcomes and measures: The primary outcome was screen-detected HCC, defined by abnormal screening-intent abdominal imaging or α-fetoprotein level within 6 months before diagnosis. Cox regression analysis was used to characterize differences in overall survival between patients with screen-detected and non-screen-detected HCC; lead-time and length-time adjustments were calculated using the Duffy parametric formula.
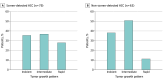
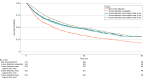
Results: Among 1313 patients with HCC (mean [SD] age, 61.7 [9.6] years; 993 male [75.6%]; 739 [56.3%] with Barcelona Clinic Liver Cancer stage 0/A disease), HCC was screen-detected in 556 (42.3%) and non-screen detected in 757 (57.7%). Patients with screen-detected HCC had higher proportions of early-stage HCC (393 patients [70.7%] vs 346 patients [45.7%]; risk ratio [RR], 1.54; 95% CI, 1.41-1.70) and curative treatment receipt (283 patients [51.1%] vs 252 patients [33.5%]; RR, 1.52; 95% CI, 1.34-1.74) compared with patients with non-screen-detected HCC. The screen-detected group had significantly lower mortality, which persisted after correcting for lead-time bias (hazard ratio, 0.75; 95% CI, 0.65-0.87) in fully adjusted models. Both groups had similar tumor doubling times (median [IQR], 3.8 [2.2-10.7] vs 5.6 [1.7-11.4] months) and proportions of indolent tumors (28 patients [35.4%] vs 24 patients [38.1%]; RR, 0.93; 95% CI, 0.60-1.43). Adjustment for length-time bias decreased survival estimates, although 3-year and 5-year survival for patients with screen-detected HCC remained longer than that for patients with non-screen-detected HCC.
Conclusions and relevance: The findings of this cohort study suggest that HCC screening is associated with reduced mortality even after accounting for lead-time and length-time biases. However, these biases should be considered in future studies.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

