Effectiveness of a Smartphone App-Based Intervention With Bluetooth-Connected Monitoring Devices and a Feedback System in Heart Failure (SMART-HF Trial): Randomized Controlled Trial
- PMID: 38683665
- PMCID: PMC11091801
- DOI: 10.2196/52075
Effectiveness of a Smartphone App-Based Intervention With Bluetooth-Connected Monitoring Devices and a Feedback System in Heart Failure (SMART-HF Trial): Randomized Controlled Trial
Abstract
Background: Current heart failure (HF) guidelines recommend a multidisciplinary approach, discharge education, and self-management for HF. However, the recommendations are challenging to implement in real-world clinical settings.
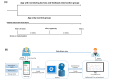
Objective: We developed a mobile health (mHealth) platform for HF self-care to evaluate whether a smartphone app-based intervention with Bluetooth-connected monitoring devices and a feedback system can help improve HF symptoms.
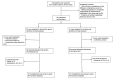
Methods: In this prospective, randomized, multicenter study, we enrolled patients 20 years of age and older, hospitalized for acute HF, and who could use a smartphone from 7 tertiary hospitals in South Korea. In the intervention group (n=39), the apps were automatically paired with Bluetooth-connected monitoring devices. The patients could enter information on vital signs, HF symptoms, diet, medications, and exercise regimen into the app daily and receive feedback or alerts on their input. In the control group (n=38), patients could only enter their blood pressure, heart rate, and weight using conventional, non-Bluetooth devices and could not receive any feedback or alerts from the app. The primary end point was the change in dyspnea symptom scores from baseline to 4 weeks, assessed using a questionnaire.
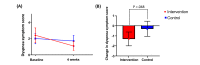
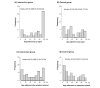
Results: At 4 weeks, the change in dyspnea symptom score from baseline was significantly greater in the intervention group than in the control group (mean -1.3, SD 2.1 vs mean -0.3, SD 2.3; P=.048). A significant reduction was found in body water composition from baseline to the final measurement in the intervention group (baseline level mean 7.4, SD 2.5 vs final level mean 6.6, SD 2.5; P=.003). App adherence, which was assessed based on log-in or the percentage of days when symptoms were first observed, was higher in the intervention group than in the control group. Composite end points, including death, rehospitalization, and urgent HF visits, were not significantly different between the 2 groups.
Conclusions: The mobile-based health platform with Bluetooth-connected monitoring devices and a feedback system demonstrated improvement in dyspnea symptoms in patients with HF. This study provides evidence and rationale for implementing mobile app-based self-care strategies and feedback for patients with HF.
Trial registration: ClinicalTrials.gov NCT05668000; https://clinicaltrials.gov/study/NCT05668000.
Keywords: heart failure; mobile applications; mobile health; mobile phone; self-care; vital sign monitoring.
©Minjae Yoon, Seonhwa Lee, Jah Yeon Choi, Mi-Hyang Jung, Jong-Chan Youn, Chi Young Shim, Jin-Oh Choi, Eung Ju Kim, Hyungseop Kim, Byung-Su Yoo, Yeon Joo Son, Dong-Ju Choi. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 29.04.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Shahim B, Kapelios CJ, Savarese G, Lund LH. Global public health burden of heart failure: an updated review. Card Fail Rev. 2023;9:e11. doi: 10.15420/cfr.2023.05. https://europepmc.org/abstract/MED/37547123 - DOI - PMC - PubMed
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32279-7 S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Park JJ, Lee CJ, Park SJ, Choi JO, Choi S, Park SM, Choi EY, Kim EJ, Yoo BS, Kang SM, Park MH, Lee J, Choi DJ. Heart failure statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021;3(4):224–236. doi: 10.36628/ijhf.2021.0023. https://europepmc.org/abstract/MED/36262554 - DOI - PMC - PubMed
-
- Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–580. doi: 10.1016/S0140-6736(17)32520-5. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(17)32520-5 S0140-6736(17)32520-5 - DOI - PMC - PubMed
-
- Cho JY, Cho DH, Youn JC, Kim D, Park SM, Jung MH, Hyun J, Choi J, Cho HJ, Park SM, Choi JO, Chung WJ, Yoo BS, Kang SM. Korean Society of Heart Failure guidelines for the management of heart failure: definition and diagnosis. Int J Heart Fail. 2023;5(2):51–65. doi: 10.36628/ijhf.2023.0009. https://europepmc.org/abstract/MED/37180563 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

