Identification of Potent and Selective Inhibitors of Acanthamoeba: Structural Insights into Sterol 14α-Demethylase as a Key Drug Target
- PMID: 38683753
- PMCID: PMC11089504
- DOI: 10.1021/acs.jmedchem.4c00303
Identification of Potent and Selective Inhibitors of Acanthamoeba: Structural Insights into Sterol 14α-Demethylase as a Key Drug Target
Abstract
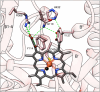
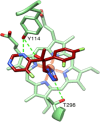
Acanthamoeba are free-living pathogenic protozoa that cause blinding keratitis, disseminated infection, and granulomatous amebic encephalitis, which is generally fatal. The development of efficient and safe drugs is a critical unmet need. Acanthamoeba sterol 14α-demethylase (CYP51) is an essential enzyme of the sterol biosynthetic pathway. Repurposing antifungal azoles for amoebic infections has been reported, but their inhibitory effects on Acanthamoeba CYP51 enzymatic activity have not been studied. Here, we report catalytic properties, inhibition, and structural characterization of CYP51 from Acanthamoeba castellanii. The enzyme displays a 100-fold substrate preference for obtusifoliol over lanosterol, supporting the plant-like cycloartenol-based pathway in the pathogen. The strongest inhibition was observed with voriconazole (1 h IC50 0.45 μM), VT1598 (0.25 μM), and VT1161 (0.20 μM). The crystal structures of A. castellanii CYP51 with bound VT1161 (2.24 Å) and without an inhibitor (1.95 Å), presented here, can be used in the development of azole-based scaffolds to achieve optimal amoebicidal effectiveness.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

