Immune signaling of Litopenaeus vannamei c-type lysozyme and its role during microsporidian Enterocytozoon hepatopenaei (EHP) infection
- PMID: 38683868
- PMCID: PMC11081493
- DOI: 10.1371/journal.ppat.1012199
Immune signaling of Litopenaeus vannamei c-type lysozyme and its role during microsporidian Enterocytozoon hepatopenaei (EHP) infection
Abstract
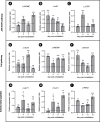
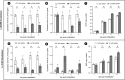
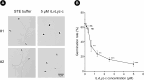
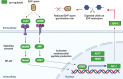
The microsporidian Enterocytozoon hepatopenaei (EHP) is a fungi-related, spore-forming parasite. EHP infection causes growth retardation and size variation in shrimp, resulting in severe economic losses. Studies on shrimp immune response have shown that several antimicrobial peptides (AMPs) were upregulated upon EHP infection. Among those highly upregulated AMPs is c-type lysozyme (LvLyz-c). However, the immune signaling pathway responsible for LvLyz-c production in shrimp as well as its function against the EHP infection are still poorly understood. Here, we characterized major shrimp immune signaling pathways and found that Toll and JAK/STAT pathways were up-regulated upon EHP infection. Knocking down of a Domeless (DOME) receptor in the JAK/STAT pathways resulted in a significant reduction of the LvLyz-c and the elevation of EHP copy number. We further elucidated the function of LvLyz-c by heterologously expressing a recombinant LvLyz-c (rLvLyz-c) in an Escherichia coli. rLvLyz-c exhibited antibacterial activity against several bacteria such as Bacillus subtilis and Vibrio parahaemolyticus. Interestingly, we found an antifungal activity of rLvLyz-c against Candida albican, which led us to further investigate the effects of rLvLyz-c on EHP spores. Incubation of the EHP spores with rLvLyz-c followed by a chitin staining showed that the signals were dramatically decreased in a dose-dependent manner, suggesting that rLvLyz-c possibly digest a chitin coat on the EHP spores. Transmission electron microscopy analysis revealed that an endospore layer, which is composed mainly of chitin, was digested by rLvLyz-c. Lastly, we observed that EHP spores that were treated with rLvLyz-c showed a significant reduction of the spore germination rate. We hypothesize that thinning of the endospore of EHP would result in altered permeability, hence affecting spore germination. This work provides insights into shrimp immune signaling pathways responsible for LvLyz-c production and its anti-EHP property. This knowledge will serve as important foundations for developing EHP control strategies.
Copyright: © 2024 Sangklai et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exit.
Figures



Similar articles
-
Role of hemocytin from Litopenaeus vannamei in immune response against microsporidian, Enterocytozoon hepatopenaei.Fish Shellfish Immunol. 2023 May;136:108710. doi: 10.1016/j.fsi.2023.108710. Epub 2023 Mar 31. Fish Shellfish Immunol. 2023. PMID: 37004896
-
Identification, characterization and heparin binding capacity of a spore-wall, virulence protein from the shrimp microsporidian, Enterocytozoon hepatopenaei (EHP).Parasit Vectors. 2018 Mar 12;11(1):177. doi: 10.1186/s13071-018-2758-z. Parasit Vectors. 2018. PMID: 29530076 Free PMC article.
-
Prophenoloxidase-activating system plays a crucial role in innate immune responses to Enterocytozoon hepatopenaei infection in shrimp Litopenaeus vannamei.Fish Shellfish Immunol. 2024 Nov;154:109925. doi: 10.1016/j.fsi.2024.109925. Epub 2024 Sep 25. Fish Shellfish Immunol. 2024. PMID: 39326689
-
Host-Parasite Interactions and Integrated Management Strategies for Ecytonucleospora Hepatopenaei Infection in Shrimp.Acta Parasitol. 2025 Mar 6;70(2):67. doi: 10.1007/s11686-025-01007-0. Acta Parasitol. 2025. PMID: 40050501 Review.
-
White feces syndrome in shrimp: Comprehensive understanding of immune system responses.Fish Shellfish Immunol. 2024 Aug;151:109704. doi: 10.1016/j.fsi.2024.109704. Epub 2024 Jun 14. Fish Shellfish Immunol. 2024. PMID: 38880362 Review.
Cited by
-
Effects of Lysophospholipids on the Antioxidant Capacity, Digestive Performance, and Intestinal Microbiota of Litopenaeus vannamei.Biology (Basel). 2025 Jan 17;14(1):90. doi: 10.3390/biology14010090. Biology (Basel). 2025. PMID: 39857320 Free PMC article.
References
-
- Anderson JL, Asche F, Garlock T. Economics of aquaculture policy and regulation. Annual Review of Resource Economics. 2019. Oct 5;11:101–23.
-
- Briggs M, Funge-Smith S, Subasinghe R, Phillips M. Introductions and movement of Penaeus vannamei and Penaeus stylirostris in Asia and the Pacific. RAP publication. 2004;10(2004):92.
-
- Flegel TW. Major viral diseases of the black tiger prawn (Penaeus monodon) in Thailand. World Journal of Microbiology and Biotechnology. 1997. Jul;13:433–42.
-
- Tourtip S, Wongtripop S, Stentiford GD, Bateman KS, Sriurairatana S, Chavadej J, et al.. Enterocytozoon hepatopenaei sp. nov.(Microsporida: Enterocytozoonidae), a parasite of the black tiger shrimp Penaeus monodon (Decapoda: Penaeidae): Fine structure and phylogenetic relationships. Journal of Invertebrate Pathology. 2009. Sep 1;102(1):21–9. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

