Neuronal innervation regulates the secretion of neurotrophic myokines and exosomes from skeletal muscle
- PMID: 38683978
- PMCID: PMC11087749
- DOI: 10.1073/pnas.2313590121
Neuronal innervation regulates the secretion of neurotrophic myokines and exosomes from skeletal muscle
Abstract
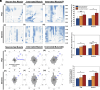
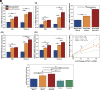
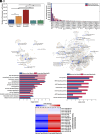
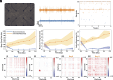
Myokines and exosomes, originating from skeletal muscle, are shown to play a significant role in maintaining brain homeostasis. While exercise has been reported to promote muscle secretion, little is known about the effects of neuronal innervation and activity on the yield and molecular composition of biologically active molecules from muscle. As neuromuscular diseases and disabilities associated with denervation impact muscle metabolism, we hypothesize that neuronal innervation and firing may play a pivotal role in regulating secretion activities of skeletal muscles. We examined this hypothesis using an engineered neuromuscular tissue model consisting of skeletal muscles innervated by motor neurons. The innervated muscles displayed elevated expression of mRNAs encoding neurotrophic myokines, such as interleukin-6, brain-derived neurotrophic factor, and FDNC5, as well as the mRNA of peroxisome-proliferator-activated receptor γ coactivator 1α, a key regulator of muscle metabolism. Upon glutamate stimulation, the innervated muscles secreted higher levels of irisin and exosomes containing more diverse neurotrophic microRNAs than neuron-free muscles. Consequently, biological factors secreted by innervated muscles enhanced branching, axonal transport, and, ultimately, spontaneous network activities of primary hippocampal neurons in vitro. Overall, these results reveal the importance of neuronal innervation in modulating muscle-derived factors that promote neuronal function and suggest that the engineered neuromuscular tissue model holds significant promise as a platform for producing neurotrophic molecules.
Keywords: exosome; innervation; myokine; neuromuscular junction; skeletal muscle.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Pedersen B. K., Febbraio M. A., Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008). - PubMed
-
- León-Ariza H. H., Mendoza-Navarrete M. P., Maldonado-Arango M. I., Botero-Rosas D. A., A systematic review of “myokines and metabolic regulation”. Apunts. Medicina de l’Esport 53, 155–162 (2018).
-
- Kim S., et al. , Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflügers Arch. 471, 491–505 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

