Phase Ib Study of Unesbulin (PTC596) Plus Dacarbazine for the Treatment of Locally Recurrent, Unresectable or Metastatic, Relapsed or Refractory Leiomyosarcoma
- PMID: 38684039
- PMCID: PMC11227303
- DOI: 10.1200/JCO.23.01684
Phase Ib Study of Unesbulin (PTC596) Plus Dacarbazine for the Treatment of Locally Recurrent, Unresectable or Metastatic, Relapsed or Refractory Leiomyosarcoma
Abstract
Purpose: This multicenter, single-arm, open-label, phase Ib study was designed to determine the recommended phase II dose (RP2D) and to evaluate the safety and preliminary efficacy of unesbulin plus dacarbazine (DTIC) in patients with advanced leiomyosarcoma (LMS).
Patients and methods: Adult subjects with locally advanced, unresectable or metastatic, relapsed or refractory LMS were treated with escalating doses of unesbulin orally twice per week in combination with DTIC 1,000 mg/m2 intravenously (IV) once every 21 days. The time-to-event continual reassessment method was used to determine the RP2D on the basis of dose-limiting toxicities (DLTs) assessed during the first two 21-day treatment cycles. All explored doses of unesbulin (200 mg up to 400 mg) were in combination with DTIC. An expansion cohort was enrolled to evaluate the safety and efficacy of unesbulin at the RP2D.
Results: Unesbulin 300 mg administered orally twice per week in combination with DTIC 1,000 mg/m2 IV once every 21 days was identified as the RP2D. On the basis of data from 27 subjects who were deemed DLT-evaluable, toxicity was higher in the unesbulin 400 mg group, with three of four subjects (75%) experiencing DLTs versus one of four subjects (25%) in the 200 mg group and three of 19 subjects (15.8%) in the 300 mg group. The most commonly reported DLTs and treatment-related grade 3 and 4 adverse events were thrombocytopenia and neutropenia. At the RP2D, seven subjects who were efficacy evaluable achieved partial response for an objective response rate of 24.1%.
Conclusion: Unesbulin 300 mg twice per week plus DTIC 1,000 mg/m2 once every 21 days was identified as the RP2D, demonstrating a favorable benefit-risk profile in a heavily pretreated population of adults with advanced LMS.
Trial registration: ClinicalTrials.gov NCT03761095.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
This author is an Associate Editor for
No other potential conflicts of interest were reported.
Figures

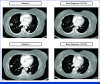
References
-
- Worhunsky DJ, Gupta M, Gholami S, et al. : Leiomyosarcoma: One disease or distinct biologic entities based on site of origin? J Surg Oncol 111:808-812, 2015 - PubMed
-
- Bessen T, Caughey GE, Shakib S, et al. : A population-based study of soft tissue sarcoma incidence and survival in Australia: An analysis of 26,970 cases. Cancer Epidemiol 63:101590, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

