Cost-effectiveness of methenamine hippurate compared with antibiotic prophylaxis for the management of recurrent urinary tract infections in secondary care: a multicentre, open-label, randomised, non-inferiority trial
- PMID: 38684270
- PMCID: PMC11086477
- DOI: 10.1136/bmjopen-2023-074445
Cost-effectiveness of methenamine hippurate compared with antibiotic prophylaxis for the management of recurrent urinary tract infections in secondary care: a multicentre, open-label, randomised, non-inferiority trial
Abstract
Objectives: To estimate the cost-effectiveness of methenamine hippurate compared with antibiotic prophylaxis in the management of recurrent urinary tract infections.
Design: Multicentre, open-label, randomised, non-inferiority trial.
Setting: Eight centres in the UK, recruiting from June 2016 to June 2018.
Participants: Women aged ≥18 years with recurrent urinary tract infections, requiring prophylactic treatment.
Interventions: Women were randomised to receive once-daily antibiotic prophylaxis or twice-daily methenamine hippurate for 12 months. Treatment allocation was not masked and crossover between arms was allowed.
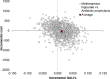
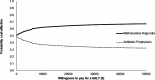
Primary and secondary outcome measures: The primary economic outcome was the incremental cost per quality-adjusted life year (QALY) gained at 18 months. All costs were collected from a UK National Health Service perspective. QALYs were estimated based on responses to the EQ-5D-5L administered at baseline, 3, 6, 9, 12 and 18 months. Incremental costs and QALYs were estimated using an adjusted analysis which controlled for observed and unobserved characteristics. Stochastic sensitivity analysis was used to illustrate uncertainty on a cost-effectiveness plane and a cost-effectiveness acceptability curve. A sensitivity analysis, not specified in the protocol, considered the costs associated with antibiotic resistance.
Results: Data on 205 participants were included in the economic analysis. On average, methenamine hippurate was less costly (-£40; 95% CI: -684 to 603) and more effective (0.014 QALYs; 95% CI: -0.05 to 0.07) than antibiotic prophylaxis. Over the range of values considered for an additional QALY, the probability of methenamine hippurate being considered cost-effective ranged from 51% to 67%.
Conclusions: On average, methenamine hippurate was less costly and more effective than antibiotic prophylaxis but these results are subject to uncertainty. Methenamine hippurate is more likely to be considered cost-effective when the benefits of reduced antibiotic use were included in the analysis.
Trial registration number: ISRCTN70219762.
Keywords: HEALTH ECONOMICS; Quality of Life; Urinary tract infections.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CH reports personal fees from Astellas Pharma (Tokyo, Japan), Medtronic plc (Dublin, Ireland), Allergan Ltd (Dublin, Ireland), GlaxoSmithKline plc (Brentford, UK), Teleflex Medical Inc (Temecula, California, USA), Viatris (Canonsburg, Pennsylvania, USA) and grants from Medtronic plc, the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme (HTA 15/40/05) and The Urology Foundation (London, UK) outside the submitted work. LV reports being the co-ordinating editor of Cochrane Incontinence (from 2016) and being a member of NIHR HTA, Clinical Evaluation and Trials Panel from 2015 to 2018. TC reports grants from the NIHR HTA programme (HTA 16/154/01, HTA 15/130/94 and HTA 11/72/01) during the conduct of the study.
Figures
References
-
- European Association of Urology . EAU guidelines – urological infections. EAU Annual Congress. Amsterdam, the Netherlands, 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical